Difference between revisions of "deLemus"
| Line 439: | Line 439: | ||
--> | --> | ||
== '''Deep Mutational Scanning Data''' == | == '''Deep Mutational Scanning Data''' == | ||
| + | The RBD-ACE2 binding data showed that R346S, N354S, E484R and S494P are the mutations lead to increased binding affinity in all the 5 background sequence. | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
RBD-ACE2 binding affinity | RBD-ACE2 binding affinity | ||
|'''Unique Mutations''' | |'''Unique Mutations''' | ||
| + | |Date | ||
|'''Wuhan''' | |'''Wuhan''' | ||
|'''Alpha''' | |'''Alpha''' | ||
| Line 450: | Line 452: | ||
|- | |- | ||
|'''R346S''' | |'''R346S''' | ||
| + | | | ||
|0.12 | |0.12 | ||
|0.14 | |0.14 | ||
| Line 455: | Line 458: | ||
|0.03 | |0.03 | ||
|0.11 | |0.11 | ||
| + | |- | ||
| + | |'''N354S''' | ||
| + | | | ||
| + | |0.03 | ||
| + | |0.01 | ||
| + | |0.04 | ||
| + | |0.32 | ||
| + | |0.02 | ||
|- | |- | ||
|'''E484R''' | |'''E484R''' | ||
| + | | | ||
|0.06 | |0.06 | ||
|0.04 | |0.04 | ||
| Line 464: | Line 476: | ||
|- | |- | ||
|'''S494P''' | |'''S494P''' | ||
| + | | | ||
|0.33 | |0.33 | ||
|0.18 | |0.18 | ||
| Line 469: | Line 482: | ||
|0.14 | |0.14 | ||
|0.06 | |0.06 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | |+ | ||
| + | |'''Unique Mutations''' | ||
| + | |Date | ||
| + | |'''Antybody1''' | ||
| + | |'''Antybody2''' | ||
| + | |'''Antybody3''' | ||
| + | |'''Antybody4''' | ||
| + | |'''Antybody5''' | ||
| + | |- | ||
| + | |'''R346S''' | ||
| + | | | ||
| + | |COV2-2082 | ||
| + | |COV2-2096 | ||
| + | |COV2-2479 | ||
| + | |COV2-2832 | ||
| + | | | ||
| + | |- | ||
| + | |'''V445A''' | ||
| + | | | ||
| + | |COV2-2050 | ||
| + | |COV2-2094 | ||
| + | |COV2-2479 | ||
| + | |COV2-2499 | ||
| + | |COV2-2677 | ||
| + | |- | ||
| + | |'''G446I''' | ||
| + | | | ||
| + | |COV2-2096 | ||
| + | |COV2-2479 | ||
| + | |COV2-2499 | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |'''E484R''' | ||
| + | | | ||
| + | |COV2-2050 | ||
| + | |COV2-2096 | ||
| + | |COV2-2479 | ||
| + | |COV2-2832 | ||
| + | | | ||
|} | |} | ||
Revision as of 14:52, 19 May 2023
Dynamic Expedition of Leading Mutations in SARS-CoV-2 Spike Glycoproteins
The dynamic epidemiology of coronavirus disease 2019 (COVID-19) since its outbreak has been a result of the continuous evolution of its etiological agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Within the first 2 years of this pandemic, the World Health Organization (WHO) has already announced 4 variants of concern (VOC), namely alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and delta (B.1.617.2), together with numerous variants of interest (VOI). The latest lineage to be designated a VOC would be omicron (B.1.1.529),[1] from which a diverse variant soup is generated.[2] From the original BA.1 strain of November 2021 to the most recent XBB and BQ.1 strains of late 2022,[3][4] each omicron subvariant has successively proliferated and outcompeted its once dominant antecedent.[5] The emergence of all these variants has brought along many novel mutations that continue to fine-tune the fitness of the virus,[6][7] leading to its persistent global circulation. Recent emerging variant (EV) data retrieved from GISAID, as of 17 January 2023, has revealed that the top 4 most rapidly spreading lineages are the BA.1.1.22, CH.1.1, XBB.1.5, and BQ.1.1 variants, among which XBB.1.5 has been found to be especially prevalent in the US,[8] making up of more than 40% of its sequence coverage in early January 2023.
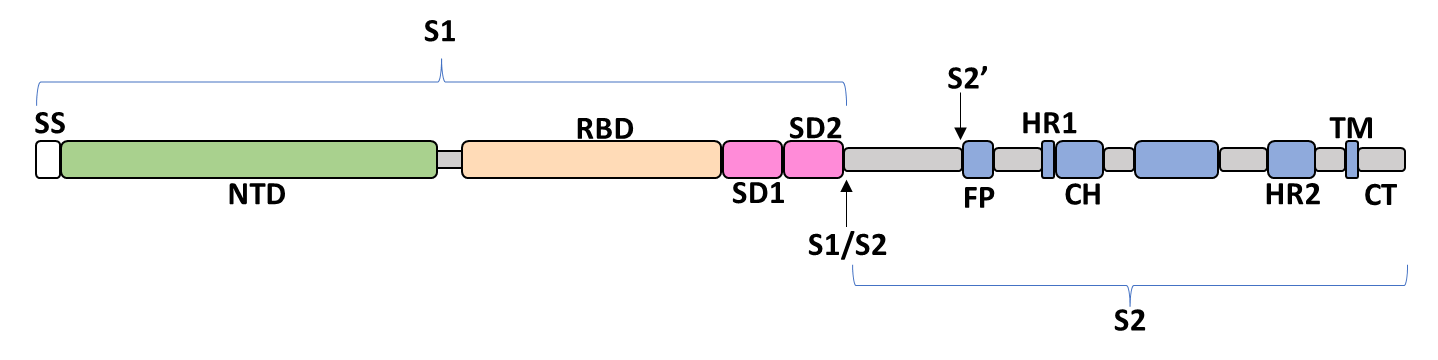
Spike Glycoprotein
The spike glycoprotein of SARS-CoV-2 is a trimeric type I viral fusion protein that binds the virus to the angiotensin-converting enzyme 2 (ACE2) receptor of a host cell.[9] It is composed of 2 subunits: the N-terminal subunit 1 (S1) and C-terminal subunit 2 (S2), within which multiple domains lie. The S1 region facilitates ACE2 binding and is made up of an N-terminal domain (NTD), a receptor-binding domain (RBD), and 2 C-terminal subdomains (CTD1 and CTD2), while the downstream S2 region is responsible for mediating virus-host cell membrane fusion.
Update
The identified leading mutations in 2023 are listed as follows [10]:

- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.05.01 - 2023.05.12
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| F456L | FD.1.1 |
*The reported mutations of detected variants are from GISAID[11]

- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.04.01 - 2023.04.21
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| H146K | XBB.1.5 & XBB.1.16 |
| M153I | XBB.2.3.3 |
| E180V | XBB.1.16 |
| K444R | XBB.1.5 |
| T478R | XBB.1.16, XBB.1.5, CH.1.1.2 & XBB.2.3 |
| F490P | XBB.2.6 |
| S494P | XBB.1.5 |
| Q613H | XBB.1.16 |
| P621S | XBB.2.3 |
| A688V | XAY.1.1.1 |
*The reported mutations of detected variants are from Cov-Lineages[12]
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
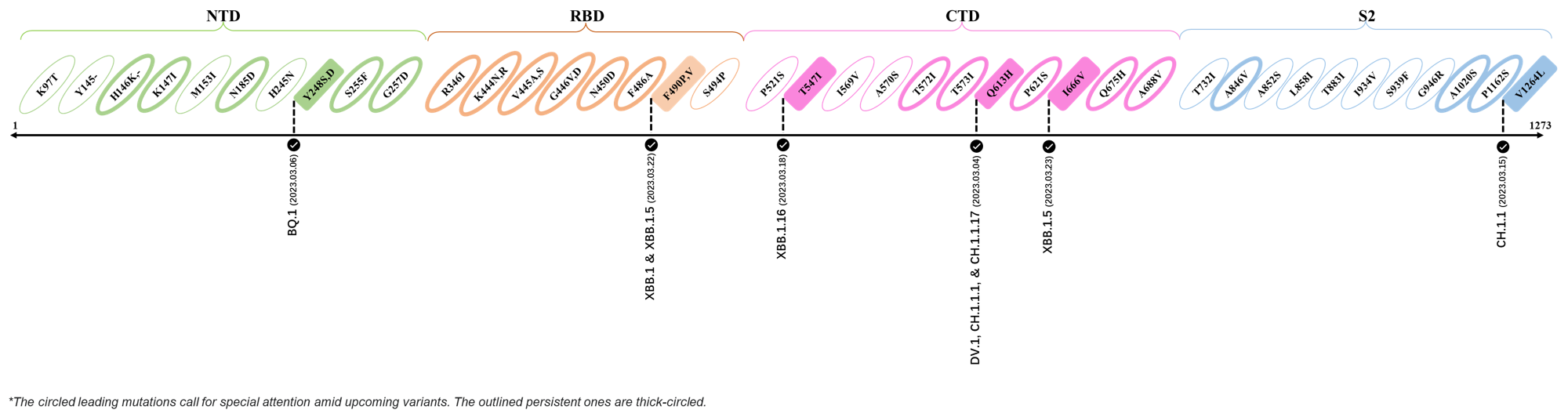
2023.03.01 - 2023.03.21
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| Y248S | BQ.1 |
| F490P | XBB.1 & XBB.1.5 |
| T547I | XBB.1.16 |
| Q613H | DV.1, CH.1.1.1 & CH.1.1.17 |
| I666V | XBB.1.5 |
| V1264L | CH.1.1 |
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
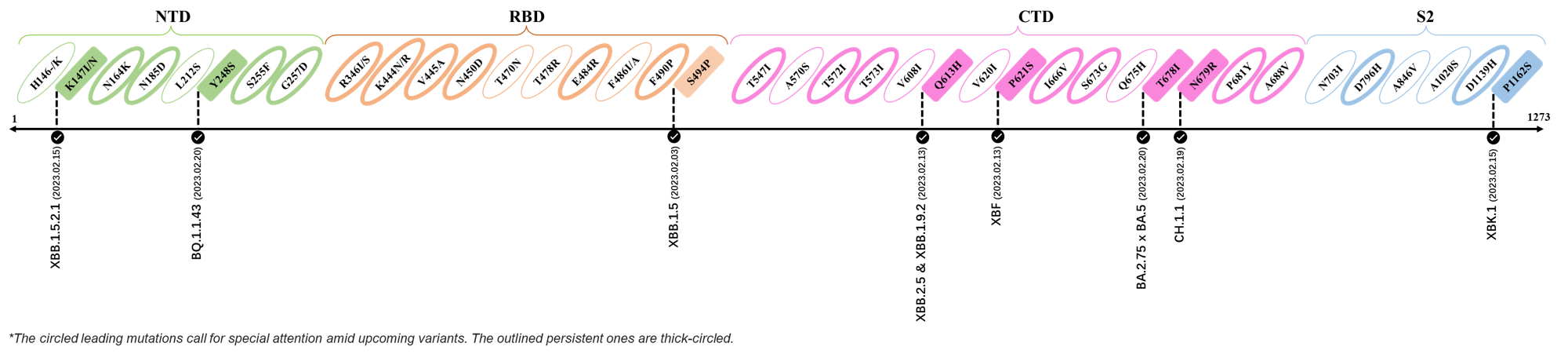
Here are the recently confirmed leading mutations.
2023.02.03 - 2023.02.20
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| K147I | XBB.1.5.2.1 |
| Y248S | BQ.1.1.43 |
| S494P | XBB.1.5 |
| Q613H | XBB.1.9.2 & XBB.2.4 |
| P612S | XBF |
| T678I | BA.2.75 x BA.5 |
| N679R | CH.1.1 |
| P1162S | XBK.1 |
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
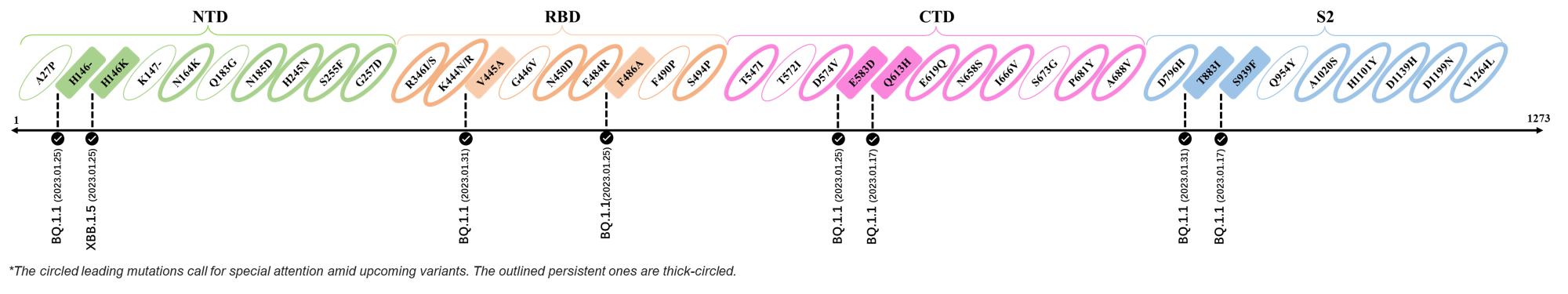
Here are the recently confirmed leading mutations.
2023.01.31
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| V445A | BQ.1.1 |
| T883I | BQ.1.1 |
2023.01.17 - 2023.01.25
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| H146- / H146K | BQ.1.1 / XBB.1.5 |
| F486A | BQ.1.1 |
| E583D | BQ.1.1 |
| Q613H | BQ.1.1 |
| S939F | BQ.1.1 |
Deep Mutational Scanning Data
The RBD-ACE2 binding data showed that R346S, N354S, E484R and S494P are the mutations lead to increased binding affinity in all the 5 background sequence.
| Unique Mutations | Date | Wuhan | Alpha | Beta | Eta | Delta |
| R346S | 0.12 | 0.14 | 0.07 | 0.03 | 0.11 | |
| N354S | 0.03 | 0.01 | 0.04 | 0.32 | 0.02 | |
| E484R | 0.06 | 0.04 | - | - | 0.11 | |
| S494P | 0.33 | 0.18 | 0.13 | 0.14 | 0.06 |
| Unique Mutations | Date | Antybody1 | Antybody2 | Antybody3 | Antybody4 | Antybody5 |
| R346S | COV2-2082 | COV2-2096 | COV2-2479 | COV2-2832 | ||
| V445A | COV2-2050 | COV2-2094 | COV2-2479 | COV2-2499 | COV2-2677 | |
| G446I | COV2-2096 | COV2-2479 | COV2-2499 | |||
| E484R | COV2-2050 | COV2-2096 | COV2-2479 | COV2-2832 |
References
- ↑ Karim, S. S. A. & Karim, Q. A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 398, 2126 (2021).
- ↑ Callaway, E. COVID ‘variant soup’ is making winter surges hard to predict. Nature 611, 213 (2022).
- ↑ Wang, Q. et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186, 279 (2023).
- ↑ Qu, P. et al. Enhanced Neutralization Resistance of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 31, 9 (2023)
- ↑ Rössler, A. et al. BA.2 and BA.5 Omicron Differ Immunologically from Both BA.1 Omicron and Pre-Omicron Variants. Nat Commun 13, 7701 (2022)
- ↑ Carabelli, A. M. et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat Rev Microbiol (2023). DOI: https://doi.org/10.1038/s41579-022-00841-7.
- ↑ Witte, L. et al. Epistasis lowers the genetic barrier to SARS-CoV-2 neutralizing antibody escape. Nat Commun 14, 302 (2023).
- ↑ Callaway, E. Coronavirus variant XBB.1.5 rises in the United States — is it a global threat? Nature 613, 222 (2023).
- ↑ Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23, 3 (2021).
- ↑ deLemus team, Analysis of Leading Mutations in SARS-CoV-2 Spike Glycoproteins (in preparation, 2023).
- ↑ GISAID https://gisaid.org/
- ↑ Cov-Lineages https://cov-lineages.org/
