Difference between revisions of "deLemus"
| Line 14: | Line 14: | ||
<tabs> | <tabs> | ||
| + | |||
| + | <tab name="2023.09"> | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-09.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | <html> | ||
| + | <style> | ||
| + | .molstar { | ||
| + | position: relative; | ||
| + | width: 80%; | ||
| + | padding-bottom: 56.25%; | ||
| + | } | ||
| + | </style> | ||
| + | <link rel="stylesheet" type="text/css" href="https://molstar.org/viewer/molstar.css" /> | ||
| + | <script type="text/javascript" src="https://molstar.org/viewer/molstar.js"></script> | ||
| + | |||
| + | <div id="viewer-7" class="molstar" style="display: block; margin-left:auto; margin-right:auto; padding-bottom: 40%;"></div> | ||
| + | <script type="text/javascript"> | ||
| + | molstar.Viewer.create('viewer-7', { | ||
| + | layoutIsExpanded: false, | ||
| + | layoutShowControls: false, | ||
| + | layoutShowRemoteState: false, | ||
| + | layoutShowSequence: true, | ||
| + | layoutShowLog: false, | ||
| + | layoutShowLeftPanel: true, | ||
| + | |||
| + | viewportShowExpand: true, | ||
| + | viewportShowSelectionMode: false, | ||
| + | viewportShowAnimation: false, | ||
| + | }).then(viewer => { | ||
| + | viewer.loadSnapshotFromUrl('https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/pdb/LM_2023_07.molx', 'molx'); | ||
| + | }); | ||
| + | </script> | ||
| + | </html> | ||
| + | |||
| + | * Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022). | ||
| + | ===<big>RBD Mutation Profile of Latest VOIs.</big>=== | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-08_VarRBD.png" alt="test for htmltag img" class="wikimg" style="display: block;width:65%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | |||
| + | </tab> | ||
<tab name="2023.08"> | <tab name="2023.08"> | ||
| Line 73: | Line 111: | ||
''*The reported mutations of detected variants are from Cov-Lineages<ref name="Cov-Lineages" />'' | ''*The reported mutations of detected variants are from Cov-Lineages<ref name="Cov-Lineages" />'' | ||
</br> | </br> | ||
| − | |||
| − | |||
</tab> | </tab> | ||
Revision as of 16:21, 5 September 2023
Dynamic Expedition of Leading Mutations in SARS-CoV-2 Spike Glycoproteins
The dynamic epidemiology of coronavirus disease 2019 (COVID-19) since its outbreak has been a result of the continuous evolution of its etiological agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Within the first 2 years of this pandemic, the World Health Organization (WHO) has already announced 4 variants of concern (VOC), namely alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and delta (B.1.617.2), together with numerous variants of interest (VOI). The latest lineage to be designated a VOC would be omicron (B.1.1.529),[1] from which a diverse variant soup is generated.[2] From the original BA.1 strain of November 2021 to the most recent XBB and BQ.1 strains of late 2022,[3][4] each omicron subvariant has successively proliferated and outcompeted its once dominant antecedent.[5] The emergence of all these variants has brought along many novel mutations that continue to fine-tune the fitness of the virus,[6][7] leading to its persistent global circulation. Recent emerging variant (EV) data retrieved from GISAID, as of 17 January 2023, has revealed that the top 4 most rapidly spreading lineages are the BA.1.1.22, CH.1.1, XBB.1.5, and BQ.1.1 variants, among which XBB.1.5 has been found to be especially prevalent in the US,[8] making up of more than 40% of its sequence coverage in early January 2023.
Spike Glycoprotein
The spike glycoprotein of SARS-CoV-2 is a trimeric type I viral fusion protein that binds the virus to the angiotensin-converting enzyme 2 (ACE2) receptor of a host cell.[9] It is composed of 2 subunits: the N-terminal subunit 1 (S1) and C-terminal subunit 2 (S2), within which multiple domains lie. The S1 region facilitates ACE2 binding and is made up of an N-terminal domain (NTD), a receptor-binding domain (RBD), and 2 C-terminal subdomains (CTD1 and CTD2), while the downstream S2 region is responsible for mediating virus-host cell membrane fusion.
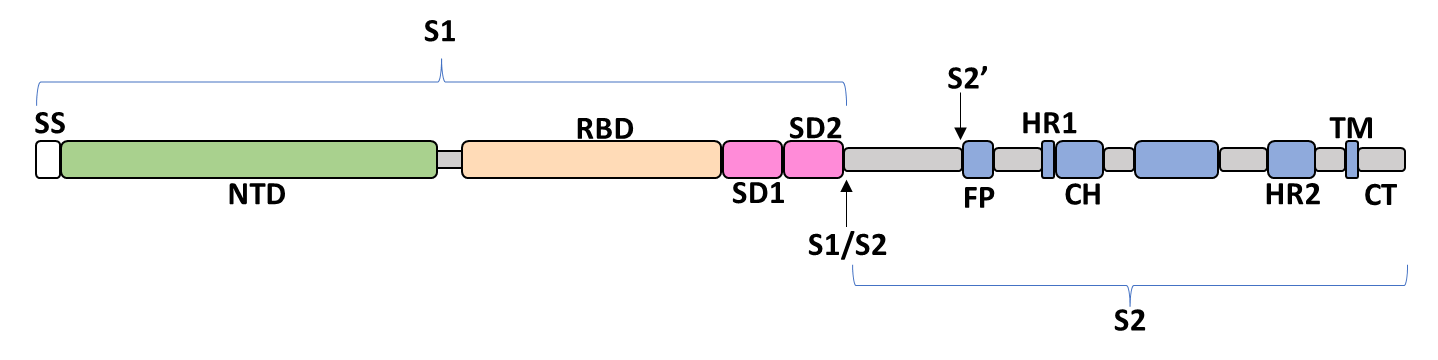
Update
The identified leading mutations in 2023 are listed as follows [10]:
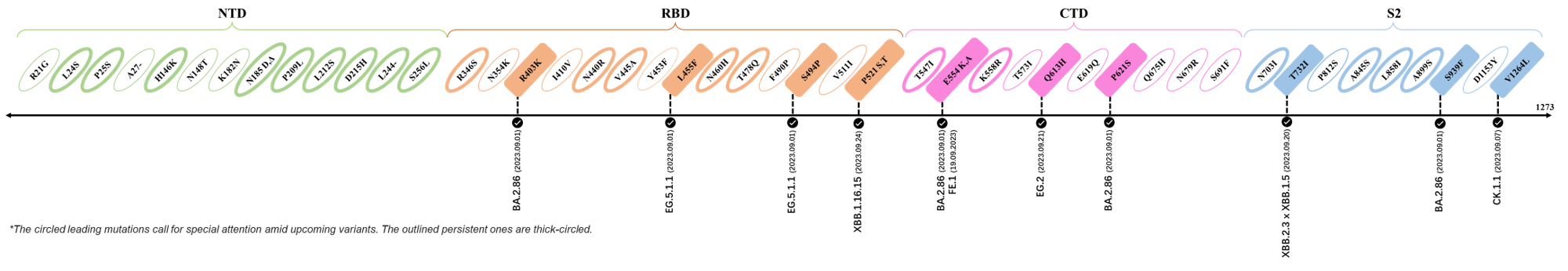
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
RBD Mutation Profile of Latest VOIs.
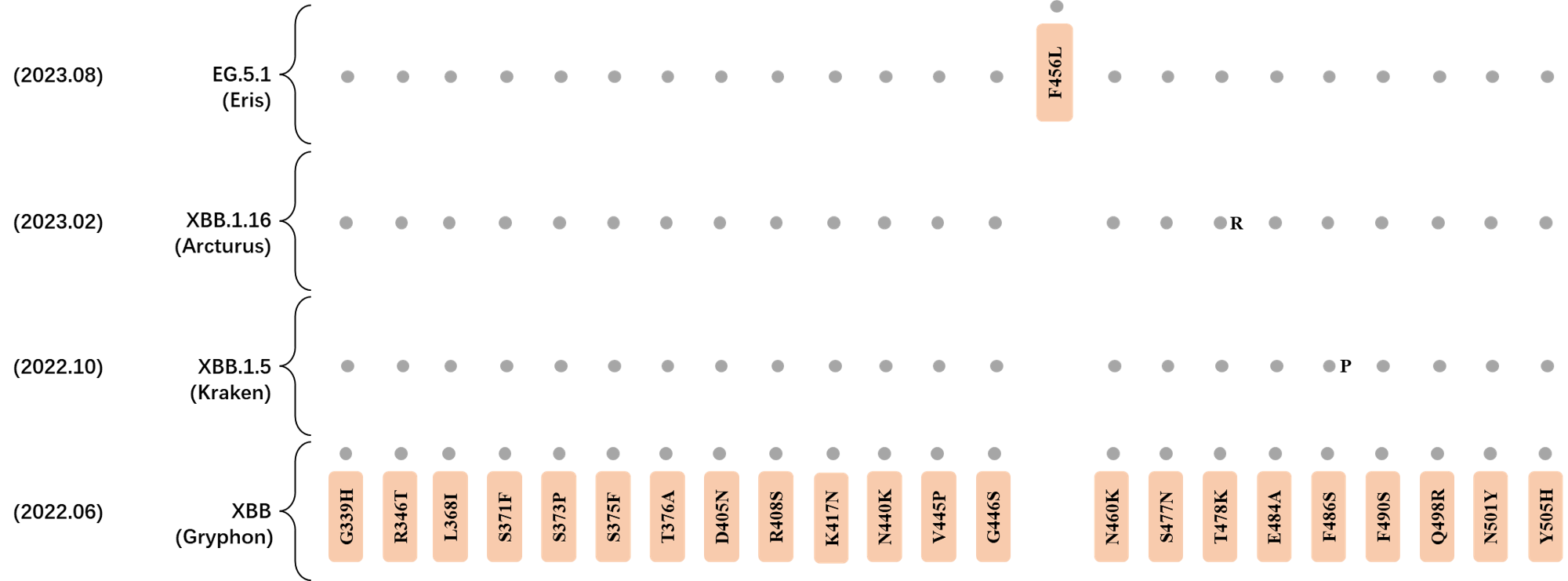
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
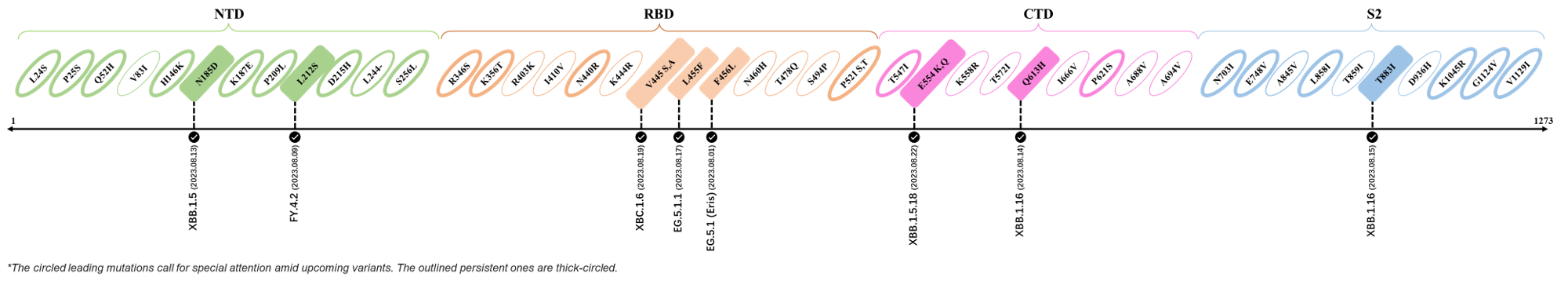
Here are the recently confirmed leading mutations.
2023.08.04 - 2023.08.22
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| N185D | XBB.1.5 |
| L212S | FY.4.2 |
| V445A | XBC.1.6 |
| L455F | EG.5.1.1 |
| F456L | EG.5.1 (Eris) |
| E554Q | XBB.1.5.18 |
| Q613H | XBB.1.16 |
| T883I | XBB.1.16 |
*The reported mutations of detected variants are from Cov-Lineages[11]
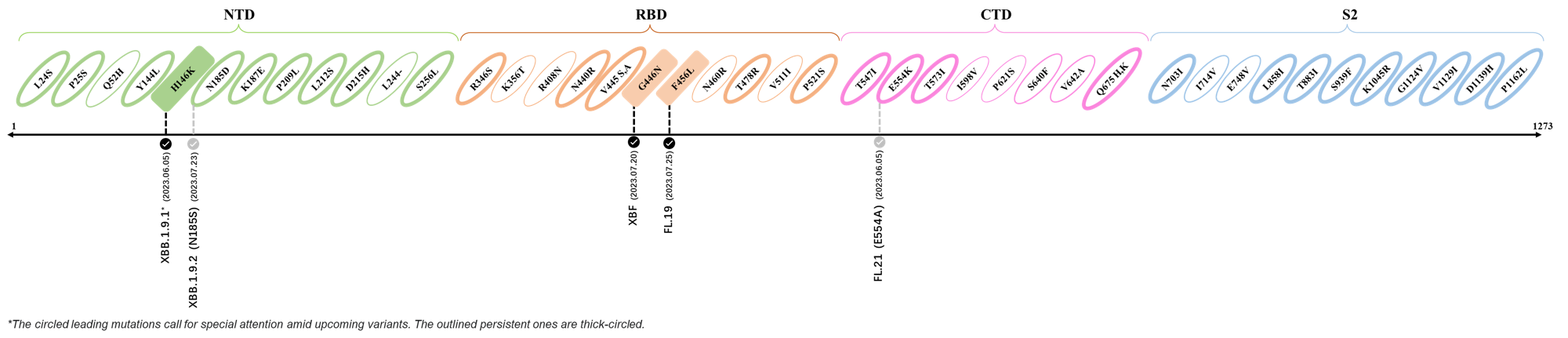
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.06.30 - 2023.07.05
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| H146K | FL.2.3 (XBB.1.9.1.2.3) |
| S446N | FL.19 |
| F456L | XBF |

- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.06.01 - 2023.06.13
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| F490P | XBB.1.9.1 |
| E554K | XBB.1.9.1 (sublineage) |
| Q675K | XBB.1.22.1 |
| L858I | CH.1.1.1 |

- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.05.01 - 2023.05.12
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| F456L | FD.1.1 & EG.5.1 (2023.08) |
| S494P | XBB.2.3 & XBB.1.1 |
| T572I | FY.1 ( XBB.1.22.1.1 ) |
*The reported mutations of detected variants are from GISAID

- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.04.01 - 2023.04.21
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| H146K | XBB.1.5 & XBB.1.16 |
| M153I | XBB.2.3.3 |
| E180V | XBB.1.16 |
| K444R | XBB.1.5 |
| T478R | XBB.1.16, XBB.1.5, CH.1.1.2 & XBB.2.3 |
| F490P | XBB.2.6 |
| S494P | XBB.1.5 |
| Q613H | XBB.1.16 |
| P621S | XBB.2.3 |
| A688V | XAY.1.1.1 |
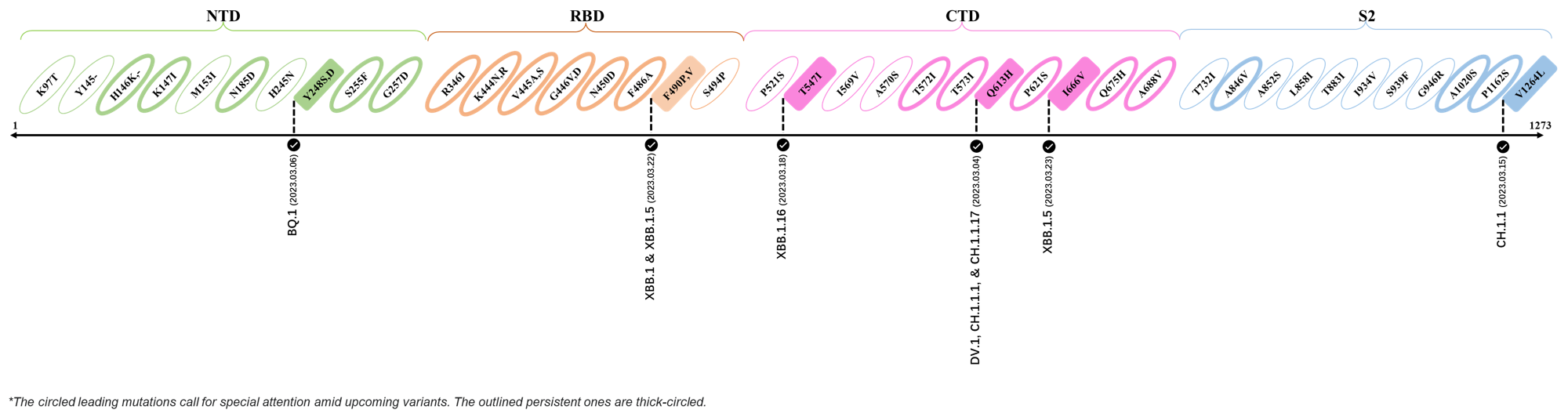
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.03.01 - 2023.03.21
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| Y248S | BQ.1 |
| F490P | XBB.1 & XBB.1.5 |
| T547I | XBB.1.16 |
| Q613H | DV.1, CH.1.1.1 & CH.1.1.17 |
| I666V | XBB.1.5 |
| V1264L | CH.1.1 |
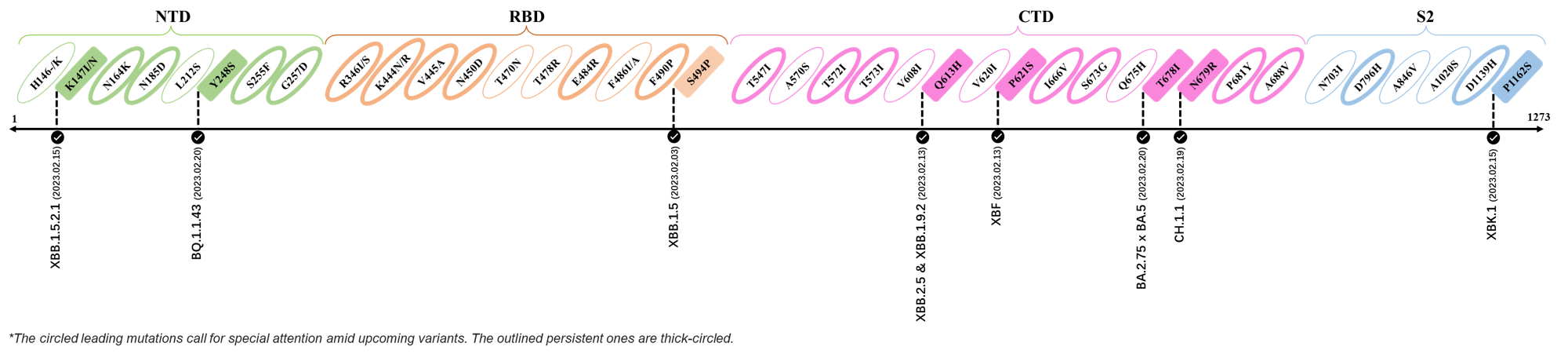
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.02.03 - 2023.02.20
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| K147I | XBB.1.5.2.1 |
| Y248S | BQ.1.1.43 |
| S494P | XBB.1.5 |
| Q613H | XBB.1.9.2 & XBB.2.4 |
| P612S | XBF |
| T678I | BA.2.75 x BA.5 |
| N679R | CH.1.1 |
| P1162S | XBK.1 |
*The reported mutations of detected variants are from GISAID[12]
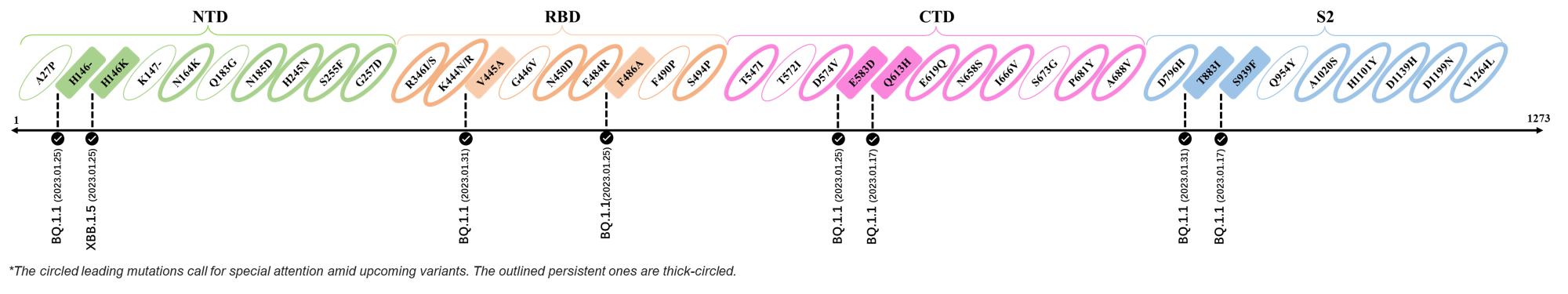
- Generated 3D structure of spike protein with highlighted leading mutations (AlphaFold2, colab version 2022).
Here are the recently confirmed leading mutations.
2023.01.31
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| V445A | BQ.1.1 |
| T883I | BQ.1.1 |
2023.01.17 - 2023.01.25
| Outlined Mutations | Confirmed in VOC/Emerging Variants |
|---|---|
| H146- / H146K | BQ.1.1 / XBB.1.5 |
| F486A | BQ.1.1 |
| E583D | BQ.1.1 |
| Q613H | BQ.1.1 |
| S939F | BQ.1.1 |
References
- ↑ Karim, S. S. A. & Karim, Q. A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 398, 2126 (2021).
- ↑ Callaway, E. COVID ‘variant soup’ is making winter surges hard to predict. Nature 611, 213 (2022).
- ↑ Wang, Q. et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186, 279 (2023).
- ↑ Qu, P. et al. Enhanced Neutralization Resistance of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 31, 9 (2023)
- ↑ Rössler, A. et al. BA.2 and BA.5 Omicron Differ Immunologically from Both BA.1 Omicron and Pre-Omicron Variants. Nat Commun 13, 7701 (2022)
- ↑ Carabelli, A. M. et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat Rev Microbiol (2023). DOI: https://doi.org/10.1038/s41579-022-00841-7.
- ↑ Witte, L. et al. Epistasis lowers the genetic barrier to SARS-CoV-2 neutralizing antibody escape. Nat Commun 14, 302 (2023).
- ↑ Callaway, E. Coronavirus variant XBB.1.5 rises in the United States — is it a global threat? Nature 613, 222 (2023).
- ↑ Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23, 3 (2021).
- ↑ deLemus team, Analysis of Leading Mutations in SARS-CoV-2 Spike Glycoproteins (in preparation, 2023).
- ↑ Cov-Lineages https://cov-lineages.org/
- ↑ GISAID https://gisaid.org/
