Difference between revisions of "Time Course"
(Created blank page) |
|||
| (198 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
| + | <big>Within the first 2 years of this pandemic, the World Health Organization (WHO) has already announced 4 variants of concern (VOC), which are the previously circulating α (B.1.1.7), β (B.1.351), γ (P.1), and δ (B.1.617.2) strains, and many other variants of interest (VOI).<ref name="WHO" /> The successive emergence of new SARS-CoV-2 variants has brought along many novel mutations. Between July and October 2022, 3 new members of the omicron (B.1.1.529) lineage have emerged, and subsequently been recognized as variants of interest (VOI) by the World Health Organization (WHO), which are the BA.2.75, XBB, and BQ.1 subvariants.<ref name="Covariants" /> Each of these VOIs has brought along an array of novel mutations in the constantly evolution of SARS-CoV-2. The time course analysis of identified leading mutations are presented here.<br /></big> | ||
| + | |||
| + | <tabs> | ||
| + | |||
| + | <tab name="2023.07"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-07a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-07b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-07c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.06"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-06a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-06b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-06c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.05"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-05a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-05b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-05c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.04"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-04a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-04b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-04c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.03"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-03a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-03b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-03c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.02"> | ||
| + | {{#tag:tab| <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-02a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"> </htmltag> |collapsed=true| name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-02b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-02c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2023.01"> | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-01a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-01b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/2023-01c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2022.08"> | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/32a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/32b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/32c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2022.06"> | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/30a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/30b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/30c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2021.08"> | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/20a.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="NTD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/20b.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="RBD"}} | ||
| + | {{#tag:tab|<htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/20c.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag>|collapsed=true|name="Other Domains"}} | ||
| + | ''Click the button to show figure.'' | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2021.03"> | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/15.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2020.12"> | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/12.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2020.11"> | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/11.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | </tab> | ||
| + | |||
| + | <tab name="2020.10"> | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/10.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | |||
| + | </tab> | ||
| + | </tabs> | ||
| + | |||
| + | ==References== | ||
| + | <references> | ||
| + | <ref name="WHO">WHO https://www.who.int/activities/tracking-SARS-CoV-2-variants</ref> | ||
| + | <ref name="Covariants">Covariants https://covariants.org/</ref> | ||
| + | </references> | ||
| + | <!-- | ||
| + | <big>Within the first 2 years of this pandemic, the World Health Organization (WHO) has already announced 4 variants of concern (VOC), which are the previously circulating α (B.1.1.7), β (B.1.351), γ (P.1), and δ (B.1.617.2) strains, and many other variants of interest (VOI). The successive emergence of new SARS-CoV-2 variants has brought along many novel mutations, most of which continually refine and improve the fitness of the virus. The identified leading mutations before the outbreak of those VOCs are listed as follows:<br /></big> | ||
| + | |||
| + | |||
| + | |||
| + | </tabs> | ||
| + | |||
| + | ==2022.08.31== | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/32.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Outlined Mutations !! Confirmed in VOC/Emerging Variants | ||
| + | |- | ||
| + | | R346T || XBB | ||
| + | |- | ||
| + | | F486S || XBB | ||
| + | |- | ||
| + | | F490S || XBB | ||
| + | |} | ||
| + | ==2022.06.30== | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/30.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Outlined Mutations !! Confirmed in VOC/Emerging Variants | ||
| + | |- | ||
| + | | F157L || BA.2.75 | ||
| + | |- | ||
| + | | G257S || BA.2.75 | ||
| + | |- | ||
| + | | G339H || BA.2.75 | ||
| + | |- | ||
| + | | N460K || BA.2.75 | ||
| + | |} | ||
| + | |||
| + | ==2021.03.31== | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/15.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Outlined Mutations !! Confirmed in VOC/Emerging Variants | ||
| + | |- | ||
| + | | T19R || δ (B.1.617.2) | ||
| + | |- | ||
| + | | E156- || δ (B.1.617.2) | ||
| + | |- | ||
| + | | F157- || δ (B.1.617.2) | ||
| + | |- | ||
| + | | R158G || δ (B.1.617.2) | ||
| + | |- | ||
| + | | L452R || δ (B.1.617.2) | ||
| + | |- | ||
| + | | T478K || δ (B.1.617.2) | ||
| + | |- | ||
| + | | P681R || δ (B.1.617.2) | ||
| + | |- | ||
| + | | D950N || δ (B.1.617.2) | ||
| + | |} | ||
| + | ==2020.10.31== | ||
| + | <htmltag tagname="img" src="https://wiki.laviebay.hkust.edu.hk/deLemus/RESEARCH_TEAMS/images/PublishedPlot/10.png" alt="test for htmltag img" class="wikimg" style="display: block;width:100%;margin-left: auto;margin-right: auto;"></htmltag> | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Outlined Mutations !! Confirmed in VOC/Emerging Variants | ||
| + | |- | ||
| + | | H69- || α (B.1.1.7) | ||
| + | |- | ||
| + | | V70- || α (B.1.1.7) | ||
| + | |- | ||
| + | | Y144- || α (B.1.1.7) | ||
| + | |- | ||
| + | | N501Y || α (B.1.1.7) | ||
| + | |- | ||
| + | | D614G || α (B.1.1.7) | ||
| + | |- | ||
| + | | P681H || α (B.1.1.7) | ||
| + | |- | ||
| + | | S982A || α (B.1.1.7) | ||
| + | |- | ||
| + | | D1118H || α (B.1.1.7) | ||
| + | |} | ||
| + | |||
| + | ===F486P/I === | ||
| + | <big>Amino acid site 486 has been exhibiting a strong mutational signal since November 2022, based on our deLemus analysis. Mutation at this site in the form of F486P is carried by the currently proliferating XBB.1.5 variant, rendering this variant with an enhanced hACE2-binding affinity when compared to its ancestor, XBB.1.<ref name="XBB.1.5"/> It is likely that the tighter receptor attachment confers quicker transmissibility for the XBB.1.5 strain, as demonstrated by its looming dominance in the US. Additionally, we have noticed another leading mutation located at the same site, F486I, which may also alter the viral fitness of SARS-CoV-2.</big> | ||
| + | |||
| + | ===K356T === | ||
| + | <big>Amino acid site 356, which corresponds to the K356T mutation, initially piqued our interest due to its persistent mutational signal since April 2022, based on our deLemus analysis. The importance of this mutation would subsequently be affirmed, as it is carried by one of the top accelerating variants, BN.1.4, according to the EV data retrieved from GISAID. In fact, a recent study has revealed that this particular mutation promotes immune evasion.<ref name=":4"/></big> <big>Moreover, it has come to our attention that this lysine-to-threonine mutation gives rise to an NXT sequon (<sub>354</sub>NRT<sub>356</sub>), which may potentially enable the generation of a novel ''N''-glycosylation site.<br /></big> | ||
| + | |||
| + | ===N460K=== | ||
| + | <big>Amino acid site 460, which corresponds to the N460K mutation, has been exhibiting a persistently strong mutational signal since February 2022, based on our deLemus analysis. Mutation at this site was first reported in the BA.2.75 strain, and was subsequently retained in the XBB and BQ.1 subvariants. The asparagine-to-lysine substitution introduces a cationic residue in the receptor binding motif (RBM) of the receptor binding domain (RBD), which increases the ACE2-binding affinity of the spike glycoprotein by enabling the formation of a new hydrogen bond with the electrostatically complementary ACE2 surface.<ref name="Zahradník"/><ref name="Makowski"/><ref name=":0"/> Moreover, this mutation grants the virus with enhanced immune evasive capability and fusogenicity for better syncytia formation.<ref name=":0" /></big> | ||
| + | |||
| + | ===R346T, L368I, and V445P=== | ||
| + | <big>Amino acid sites 346, 368, and 445 have been exhibiting strong mutational signals since the end of 2021, May 2022, and April 2022 respectively, based on our deLemus analysis. Out of these 3 sites, only the R346 residue displays significant polymorphism, as seen from how 2 different substitutions have arisen from this site, being the R346K of mu (B.1.621) and R346T of XBB; the other 2 mutations are specific to XBB. All these RBD mutations are experimentally found to confer immune evasion capabilities when carried by the spike glycoprotein,<ref name=":4" /><ref name=":2"/> of which the R346T mutation particularly impairs the binding of class 3 antibodies.<ref name=":3"/> While the immunologically effects of these mutations are not well elucidated mechanistically, structural analysis has revealed that the bulky phenylaniline residue introduced by the V445P mutation is what contributed to the weakened spike-antibody interactions.<ref name=":3" /> In addition to these immune escape functions, L368I has been characterized to increase the ACE2-binding affinity of the SARS-CoV-2 spike.<ref name=":2" /></big> | ||
| + | |||
| + | ===K444T and F486V/S=== | ||
| + | <big>Amino acid sites 444 and 486 have been exhibiting strong mutational signals since the end of 2021 and March 2022 respectively, based on our deLemus analysis. Mutation at the former site, K444T, is specific to the BQ.1 subvariant, which has been reported to hinder the binding of class 3 antibodies by abrogating its hydrogen bond and salt bridge formation with the spike glycoprotein.<ref name=":1"/> Residue at the latter location, on the other hand, has been identified to be polymorphic, as indicated by the fact that 2 major substitutions exist in this site, which are the F486V of BA.4, BA.5, and BQ.1, and F486S of XBB. The crucial role of this phenylaniline residue as both an ACE2 and antibody binding site implicates that spike glycoproteins carrying the F486V/S mutations would possess enhanced immune evasion capabilities against certain class 1 and 2 antibodies at the cost of having lowered ACE2-binding affinities.<ref name=":1" /><ref name="Tuekprakhon"/><ref name="Wang"/></big> | ||
| + | |||
| + | == References == | ||
| + | <references> | ||
| + | <ref name="XBB.1.5">Yue, C. ''et al''. Enhanced transmissibility of XBB.1.5 is contributed by both strong ACE2 binding and antibody evasion. Preprint at https://www.biorxiv.org/content/10.1101/2023.01.03.522427v2 (2023).</ref> | ||
| + | <ref name=":4">Cao, Y. ''et al.'' Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. ''Nature'' (2022). DOI:10.1038/s41586-022-05644-7</ref> | ||
| + | <ref name="Zahradník">Zahradník, J. ''et al.'' SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. ''Nat Microbiol'' '''6,''' 1188 (2021).</ref> | ||
| + | <ref name="Makowski">Makowski, E. K., Schardt, J. S., Smith, M. D. & Tessier, P. M. Mutational analysis of SARS-CoV-2 variants of concern reveals key tradeoffs between receptor affinity and antibody escape. ''PLOS Comput Biol'' '''18,''' (2022).</ref> | ||
| + | <ref name=":0">Qu, P. ''et al.'' Evasion of neutralizing antibody responses by the SARS-CoV-2 BA.2.75 variant. ''Cell Host Microbe'' '''30,''' 1518 (2022).</ref> | ||
| + | <ref name=":2">Tamura, T. ''et al.'' Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two omicron subvariants. Preprint at https://www.biorxiv.org/content/10.1101/2022.12.27.521986v1 (2022).</ref> | ||
| + | <ref name=":3">Wang, Q. ''et al.'' Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. ''Cell'' '''186,''' 279 (2023).</ref> | ||
| + | <ref name=":1">Qu, P. ''et al.'' Enhanced neutralization resistance of SARS-CoV-2 omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. ''Cell Host Microbe'' '''31,''' 9 (2023).</ref> | ||
| + | <ref name="Tuekprakhon">Tuekprakhon, A. ''et al.'' Antibody escape of SARS-CoV-2 omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. ''Cell'' '''185,''' 2422 (2022).</ref> | ||
| + | <ref name="Wang">Wang, Q. ''et al.'' Antibody evasion by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. ''Nature'' '''608,''' 603 (2022).</ref> | ||
| + | </references> | ||
| + | --> | ||
| + | [[Category:deLemus]] | ||
Latest revision as of 15:28, 4 August 2023
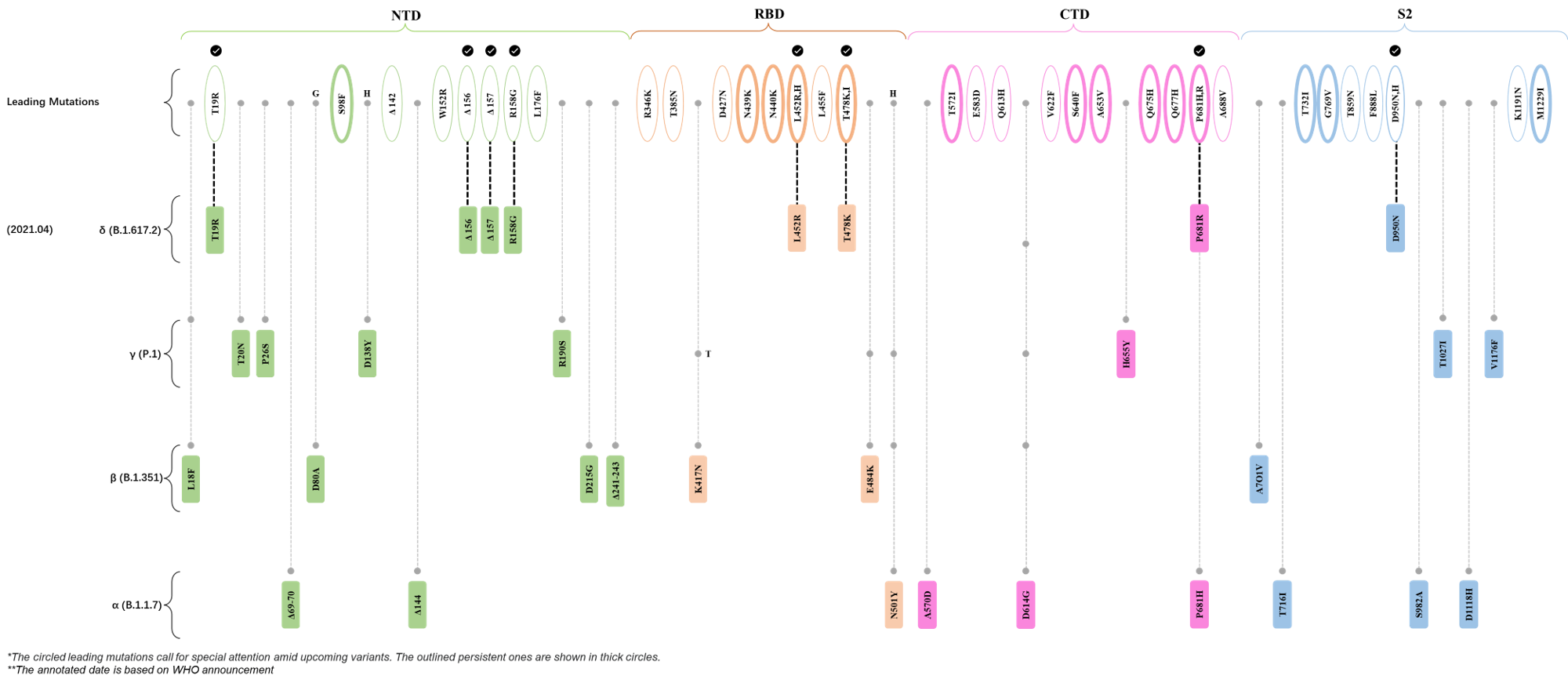
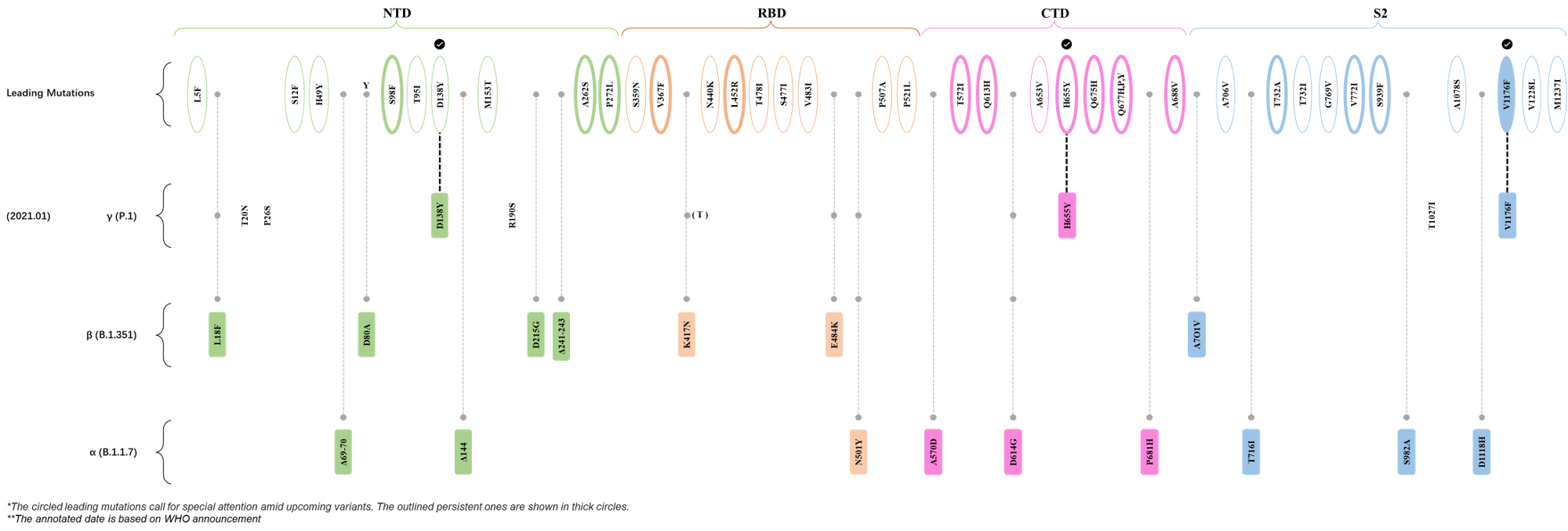
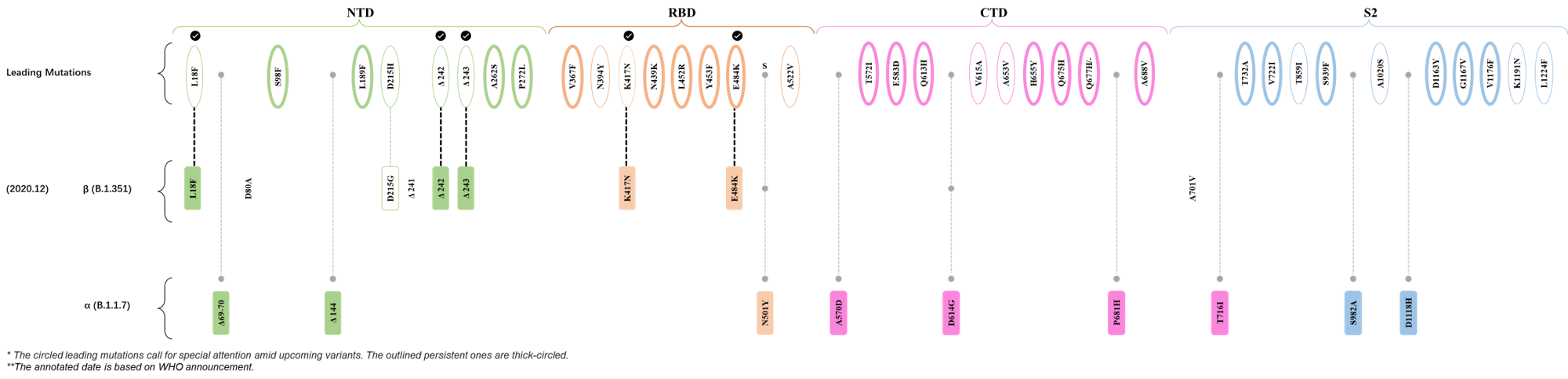
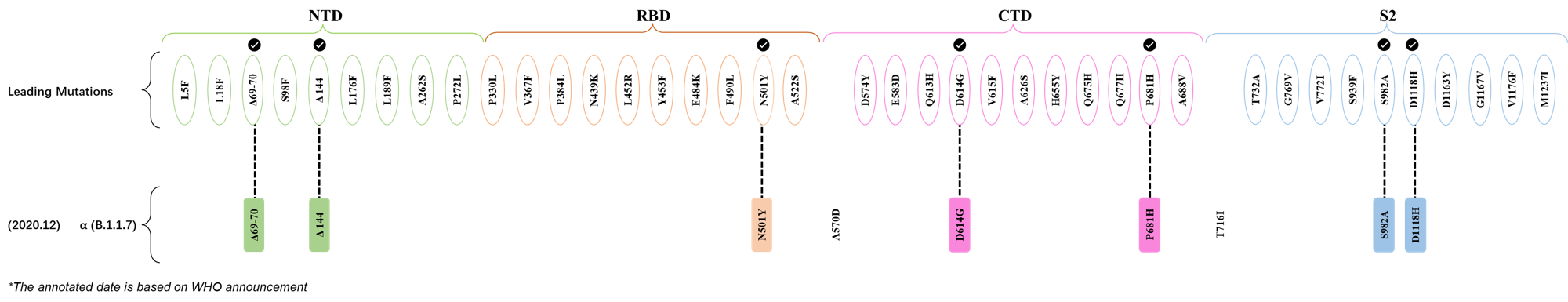
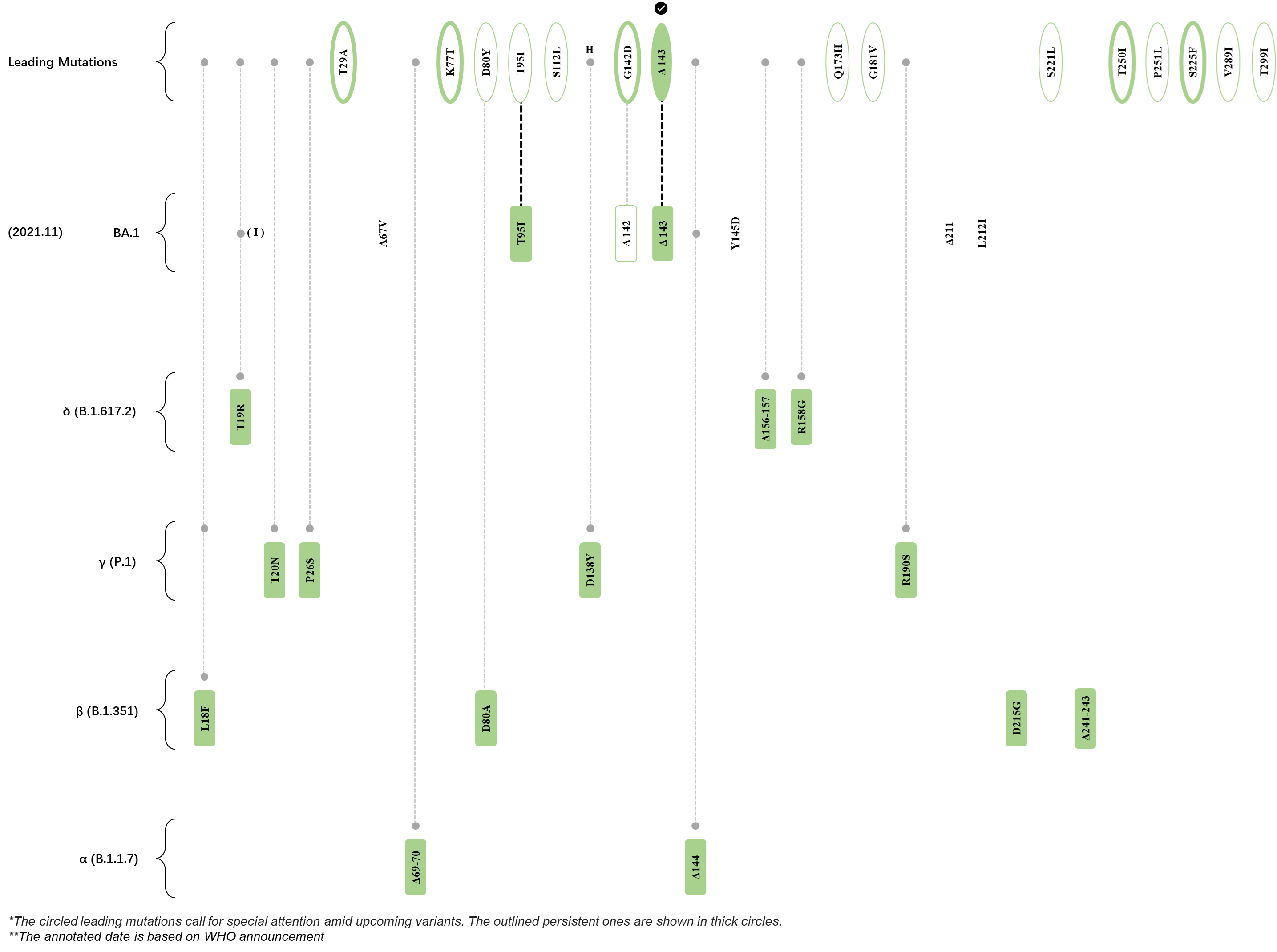
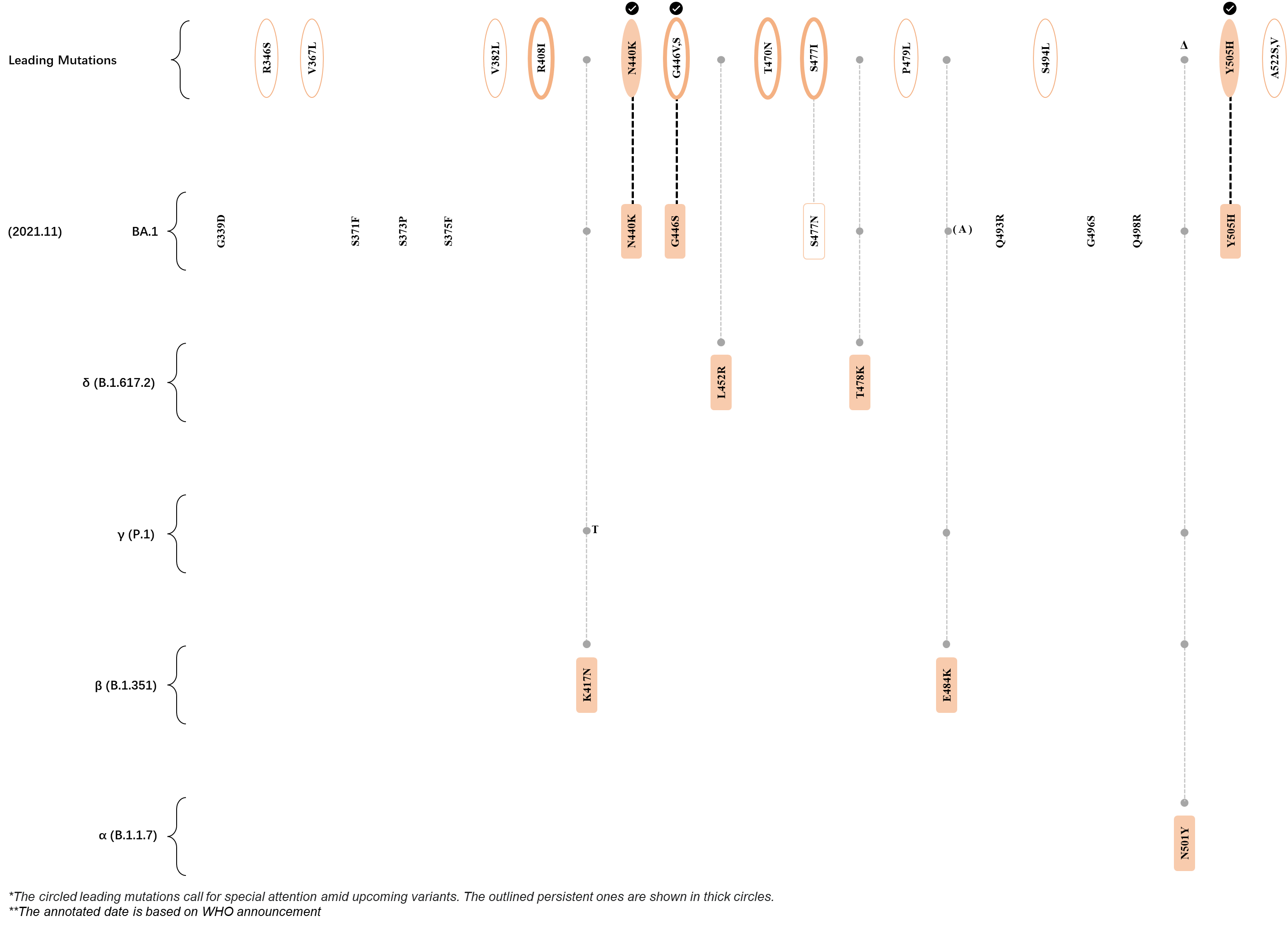
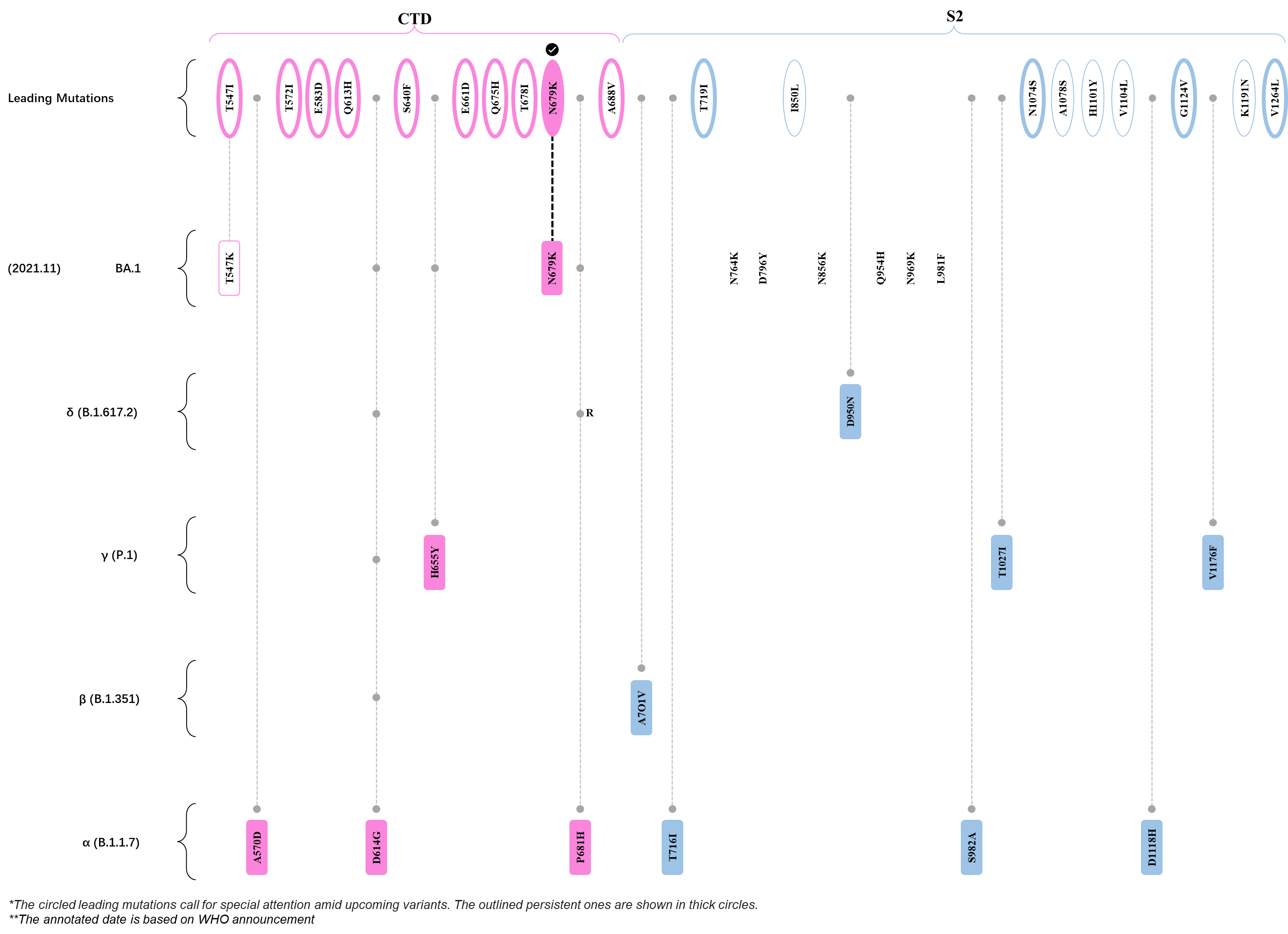
Within the first 2 years of this pandemic, the World Health Organization (WHO) has already announced 4 variants of concern (VOC), which are the previously circulating α (B.1.1.7), β (B.1.351), γ (P.1), and δ (B.1.617.2) strains, and many other variants of interest (VOI).[1] The successive emergence of new SARS-CoV-2 variants has brought along many novel mutations. Between July and October 2022, 3 new members of the omicron (B.1.1.529) lineage have emerged, and subsequently been recognized as variants of interest (VOI) by the World Health Organization (WHO), which are the BA.2.75, XBB, and BQ.1 subvariants.[2] Each of these VOIs has brought along an array of novel mutations in the constantly evolution of SARS-CoV-2. The time course analysis of identified leading mutations are presented here.
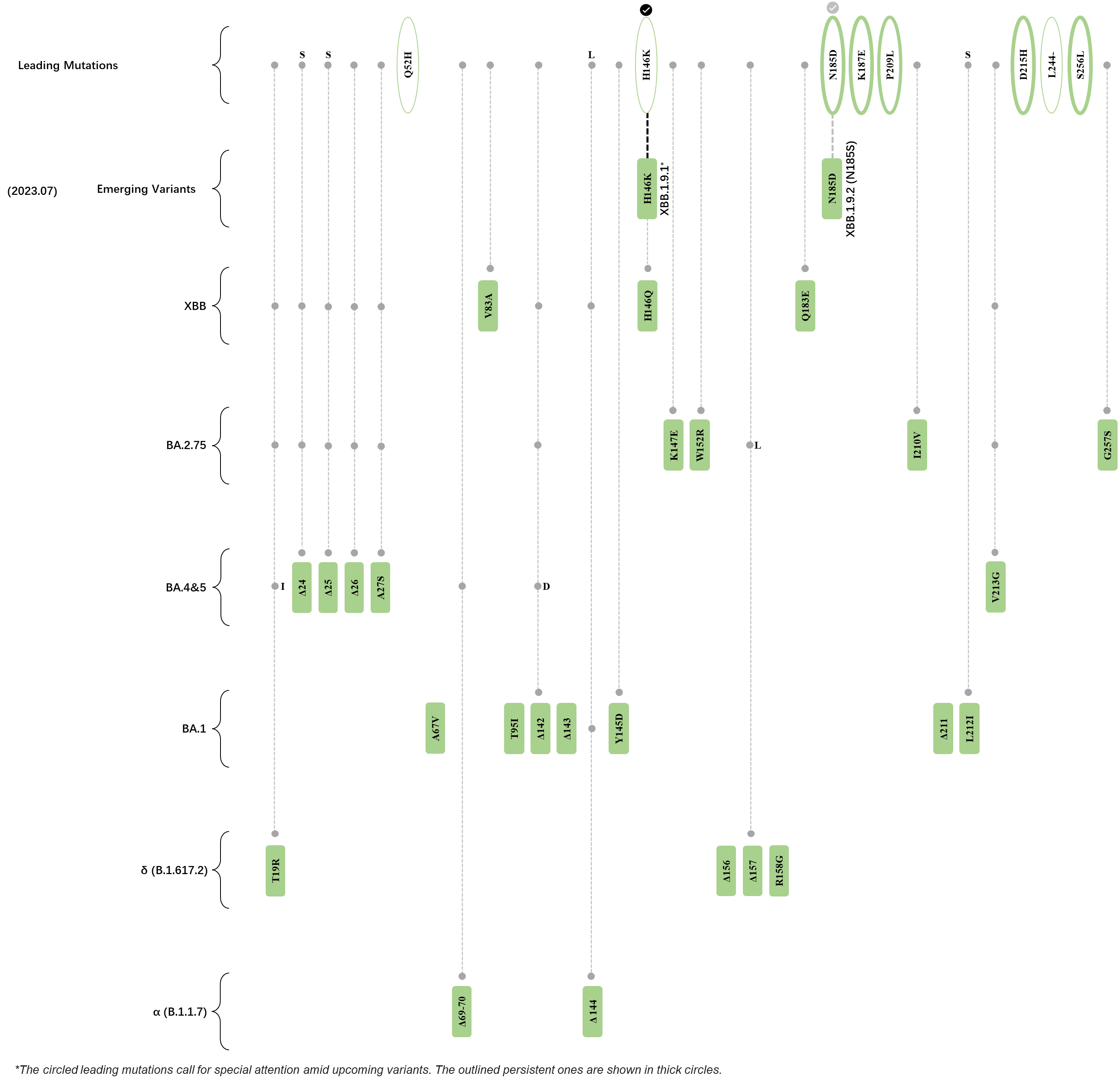
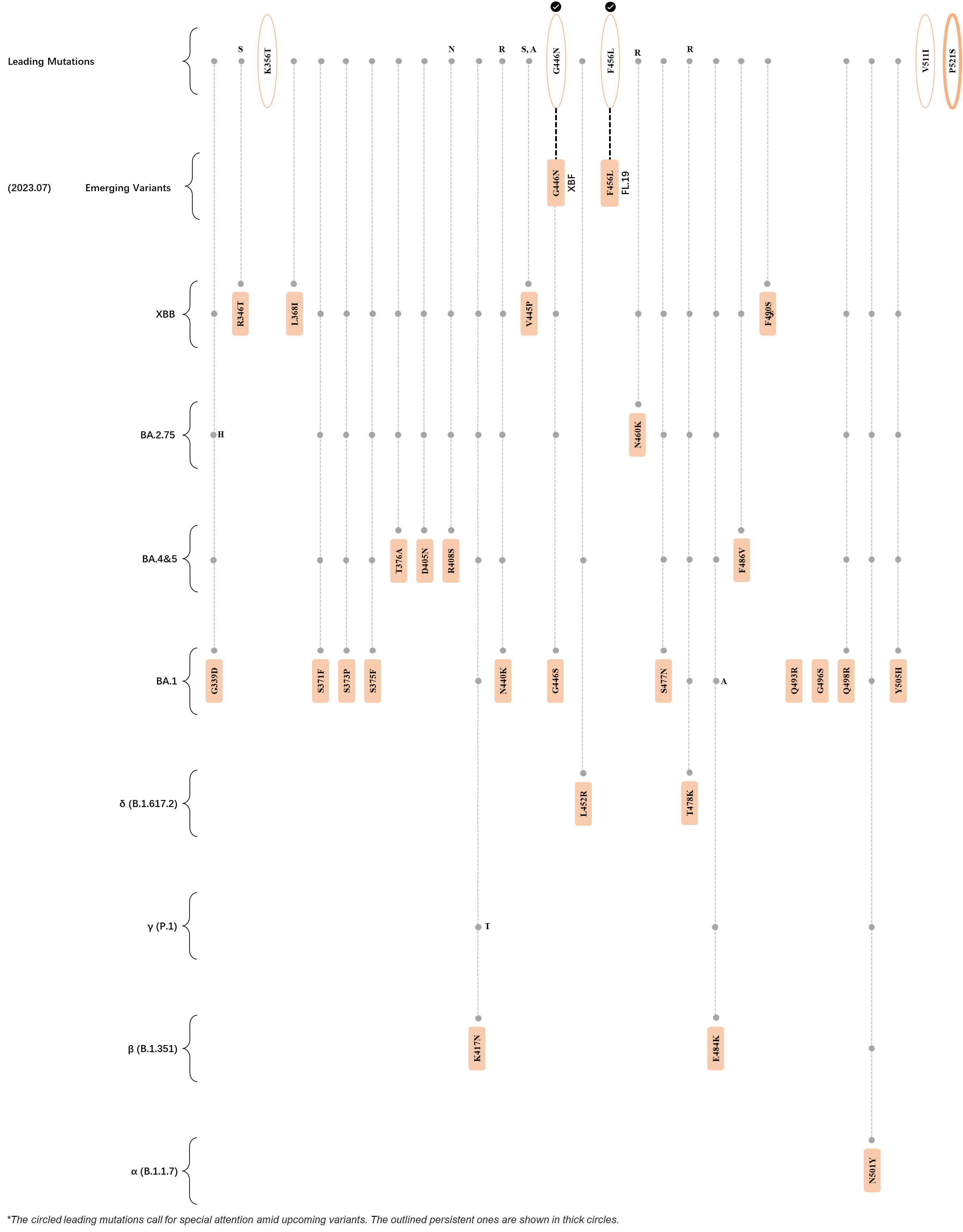
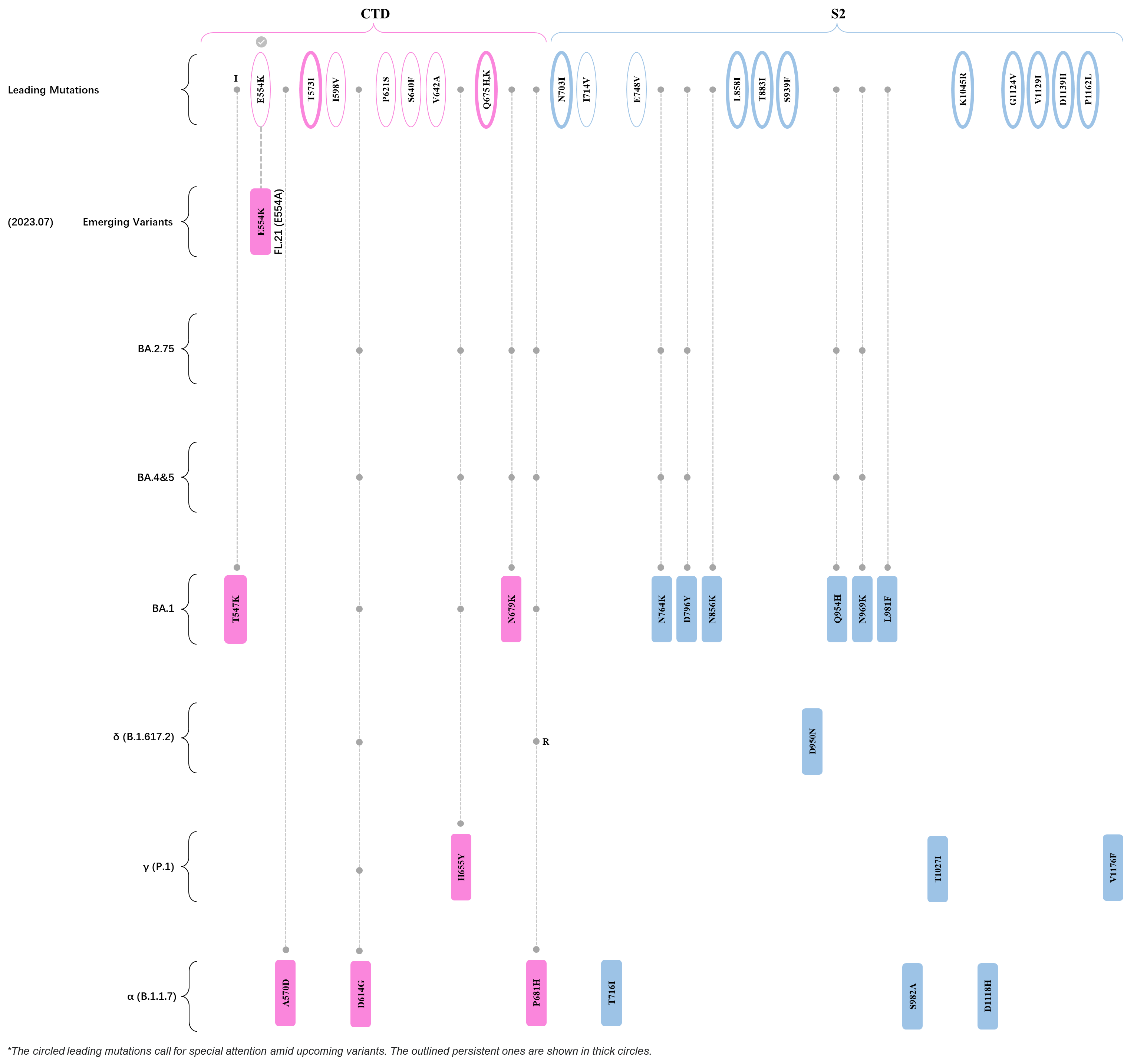
Click the button to show figure.
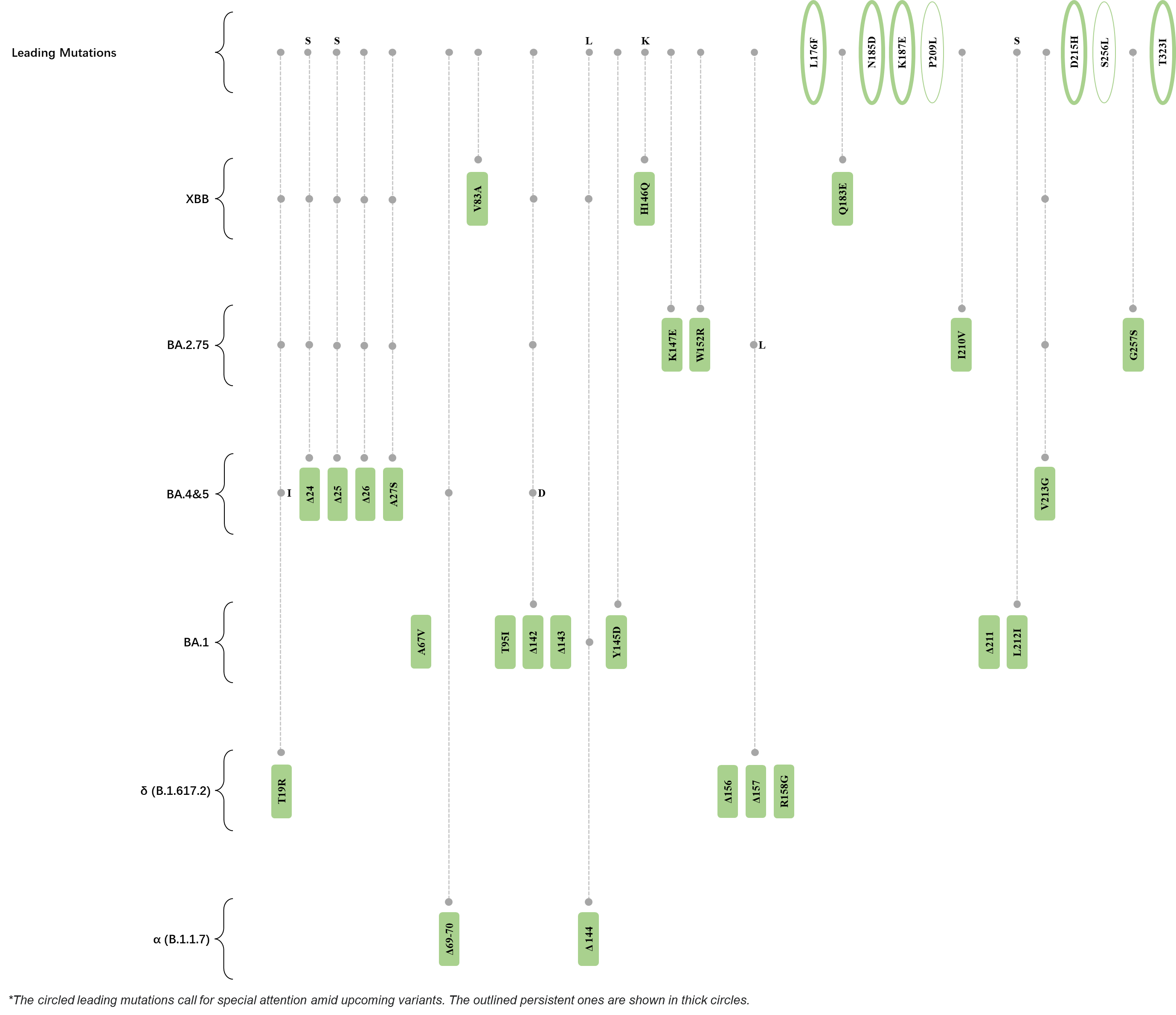
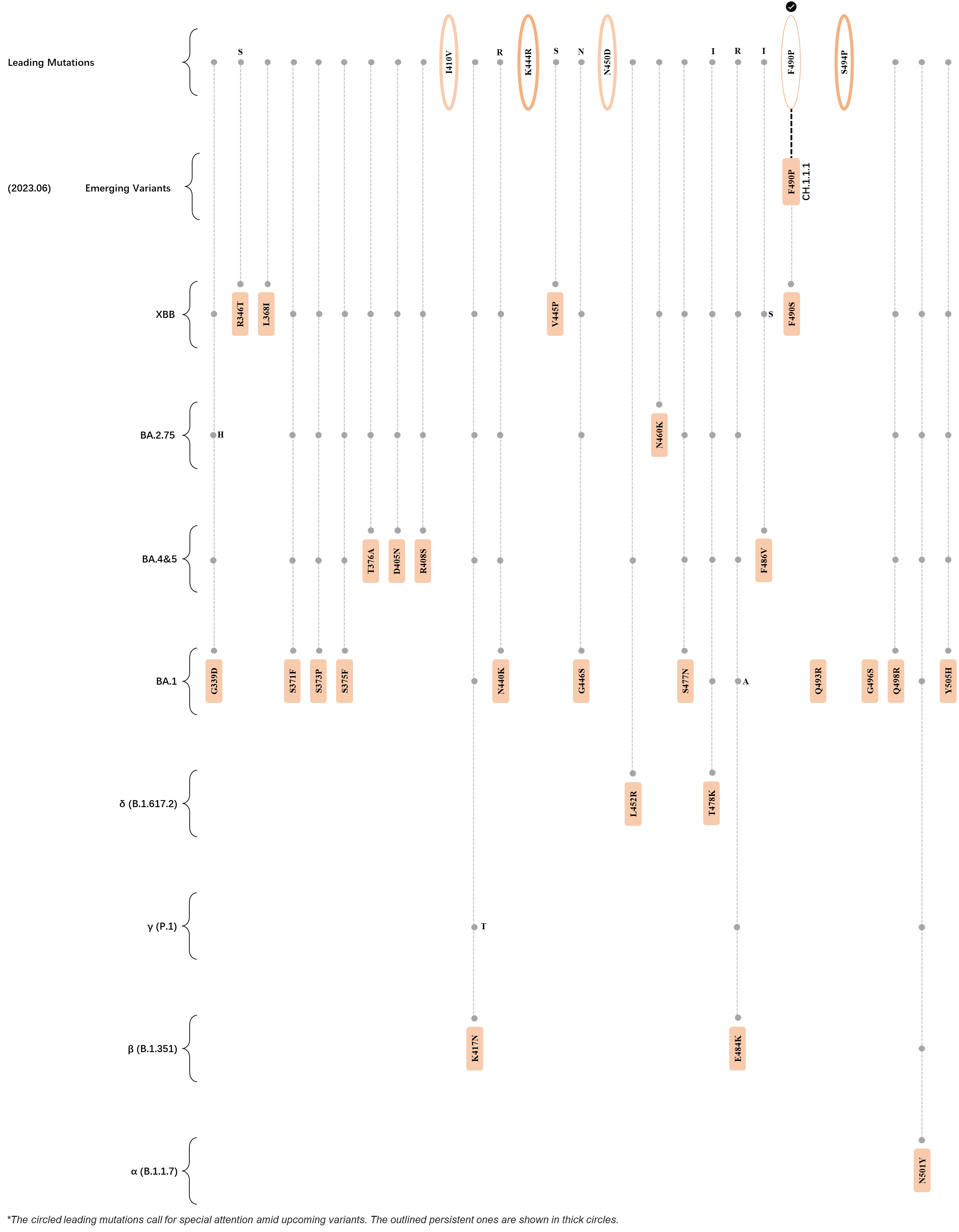
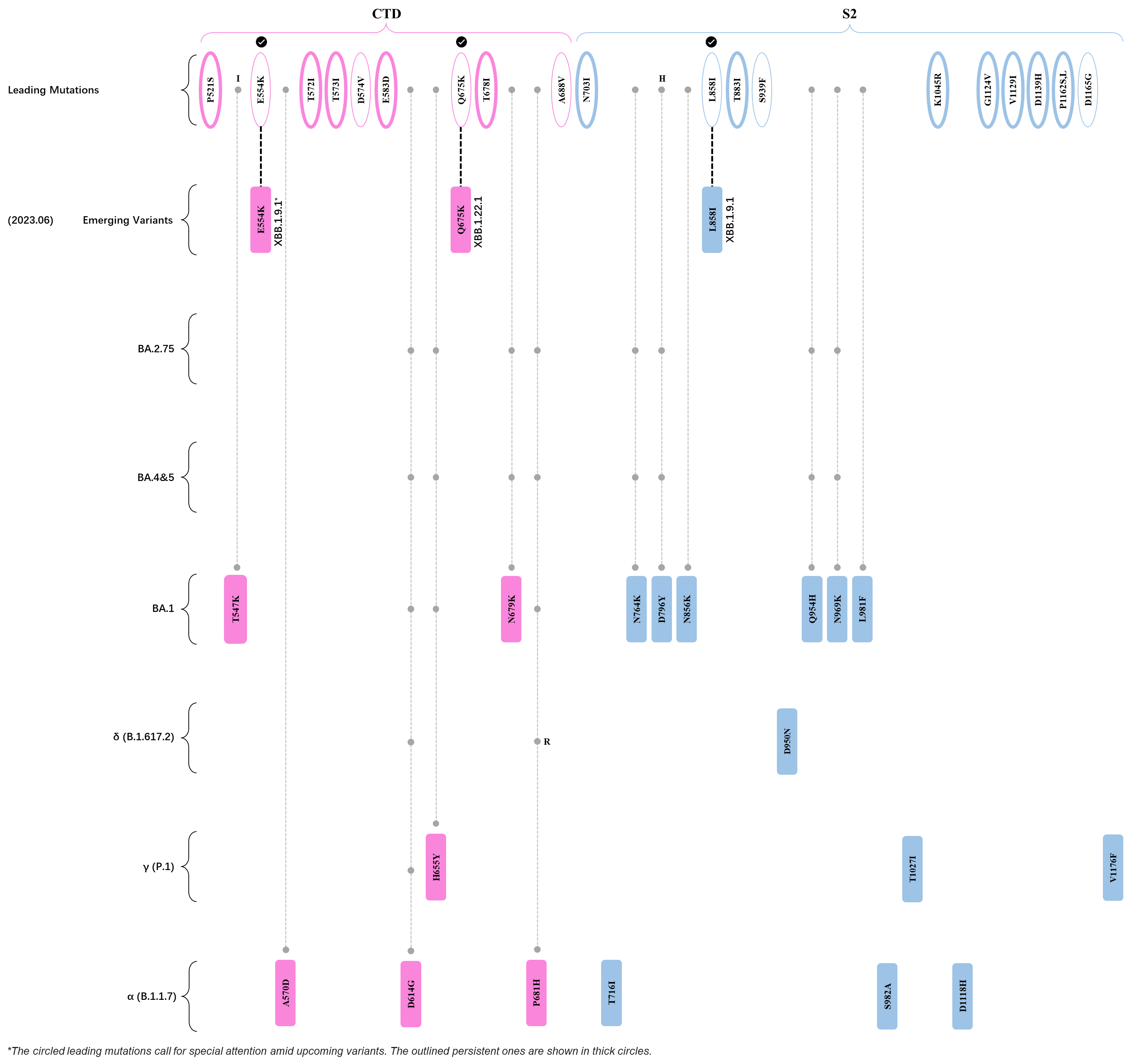
Click the button to show figure.
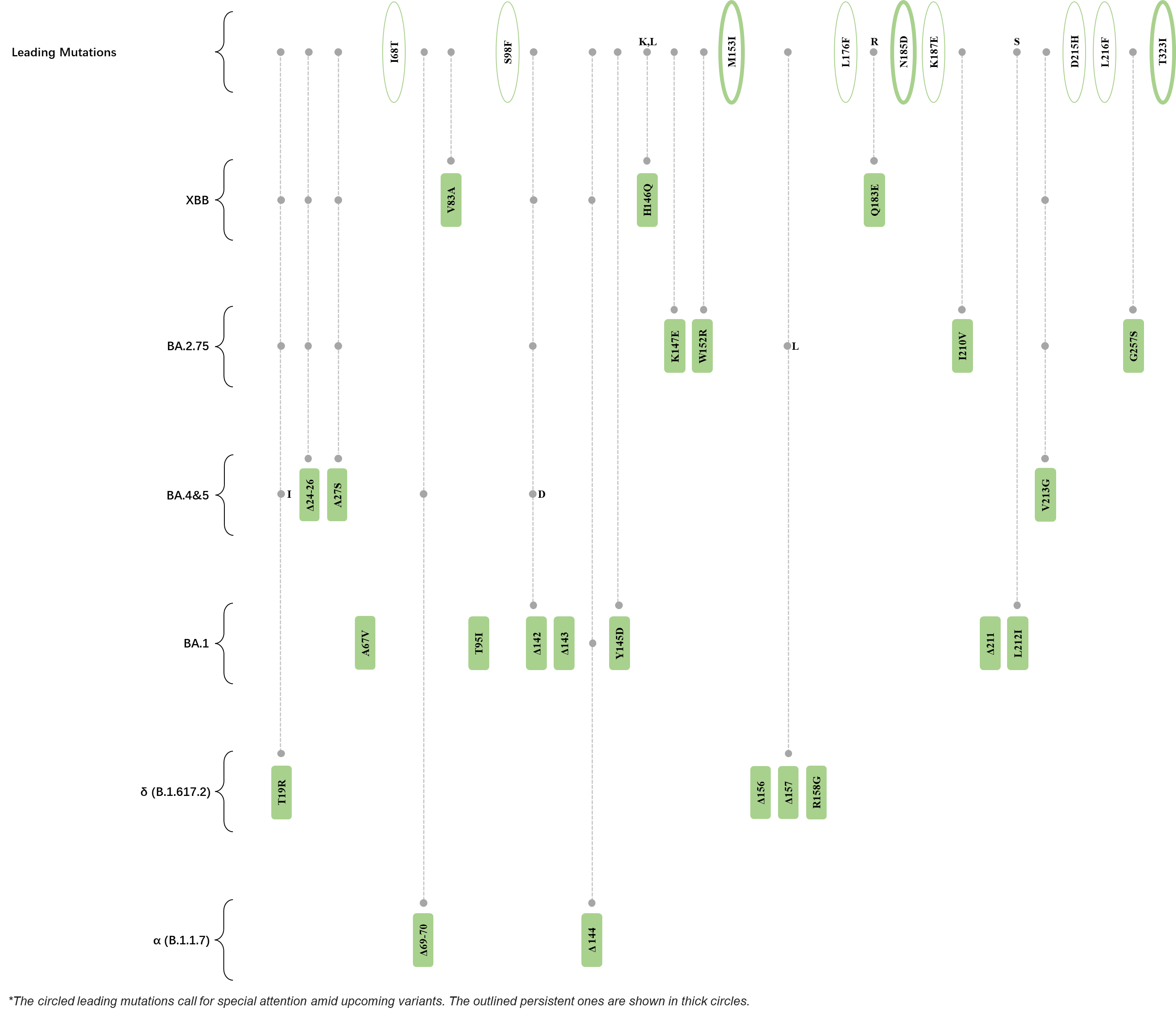


Click the button to show figure.
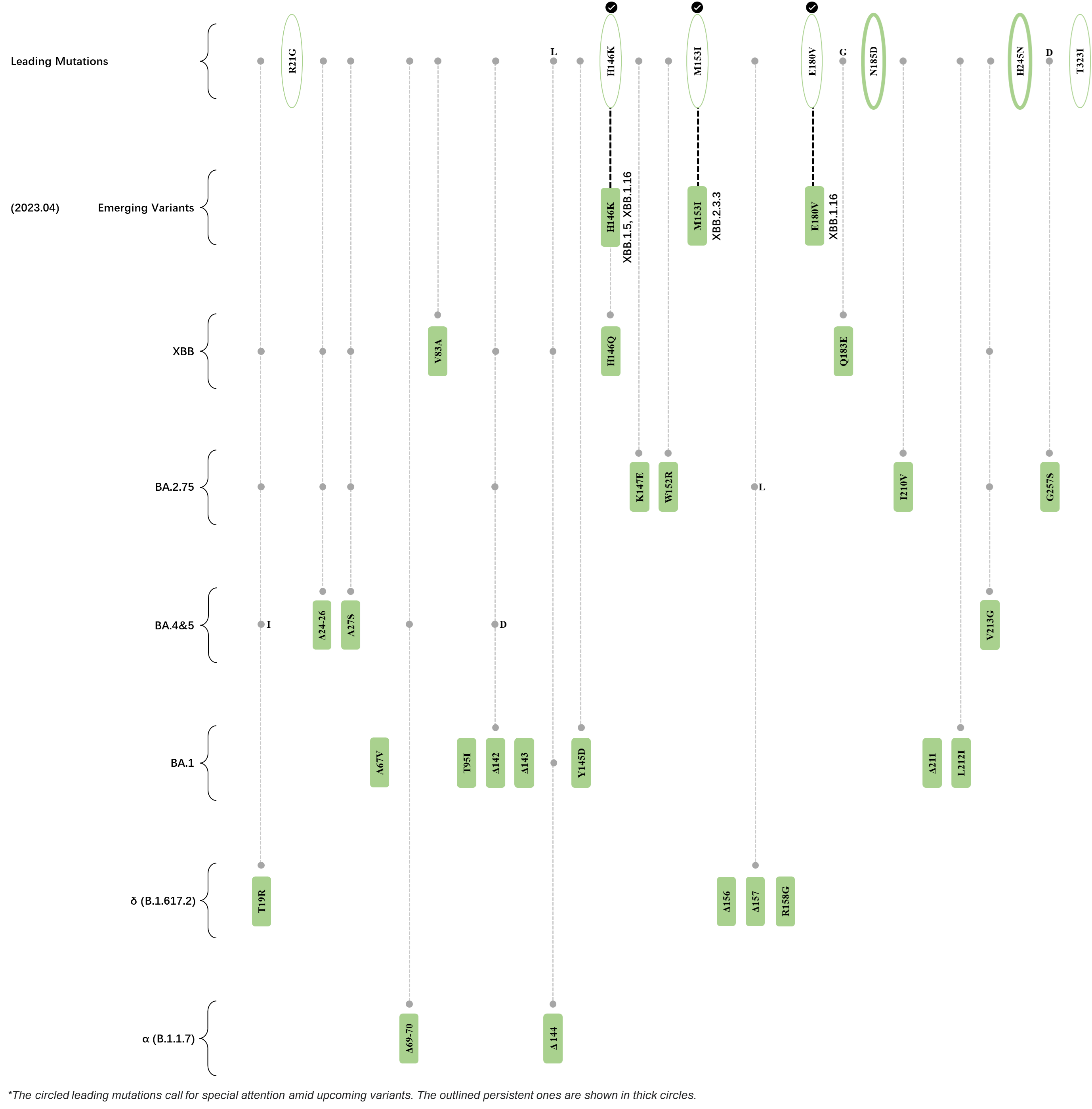
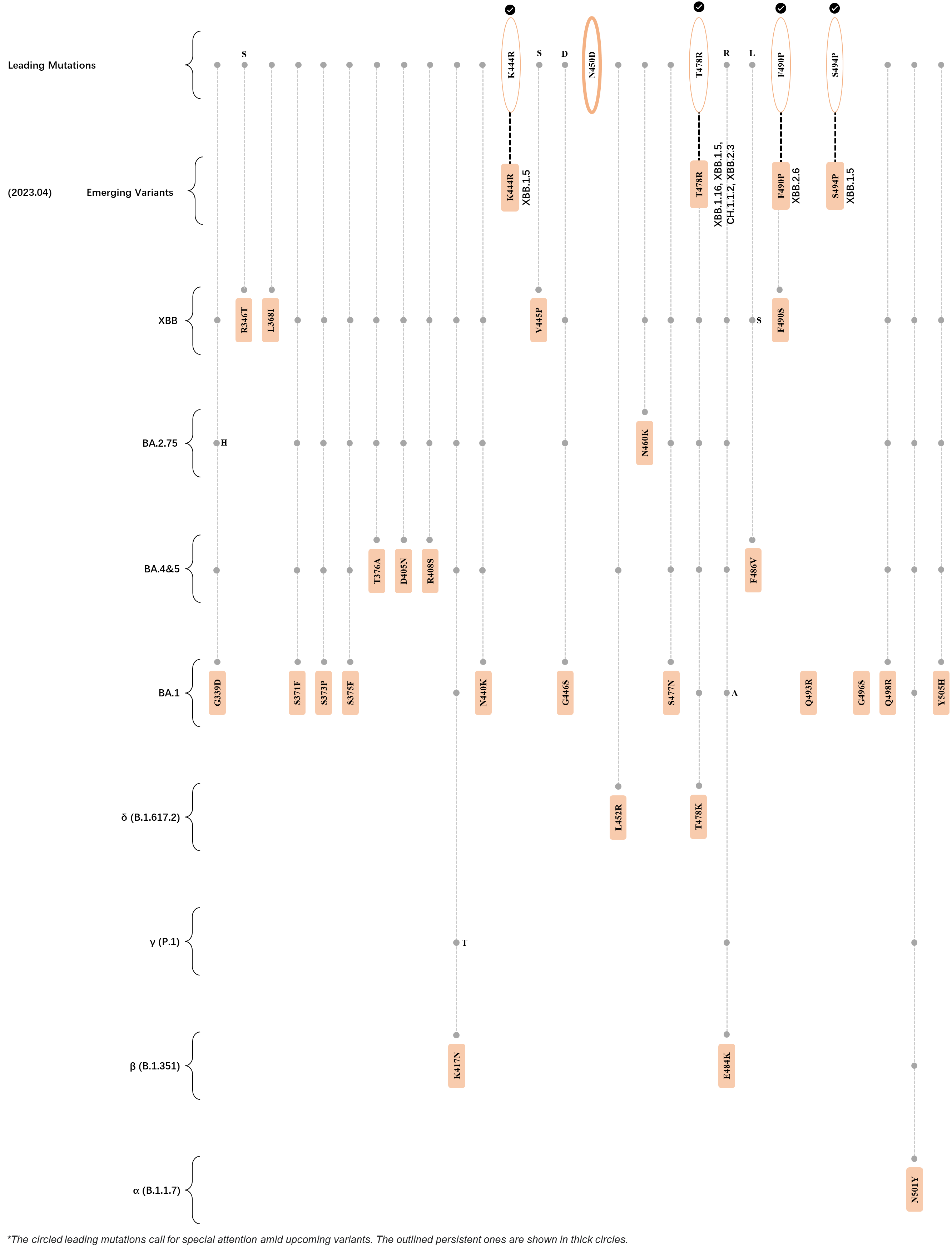
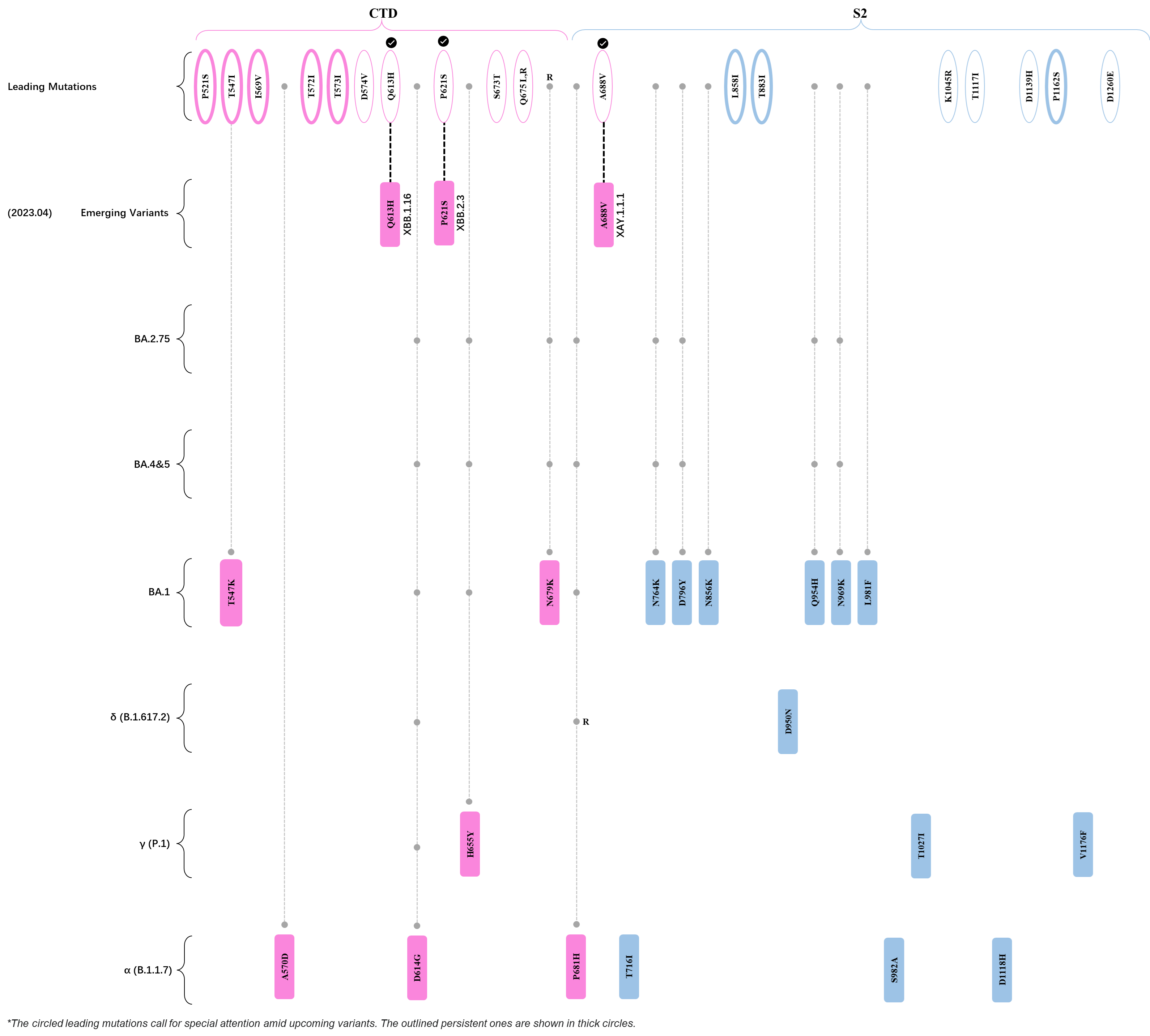
Click the button to show figure.
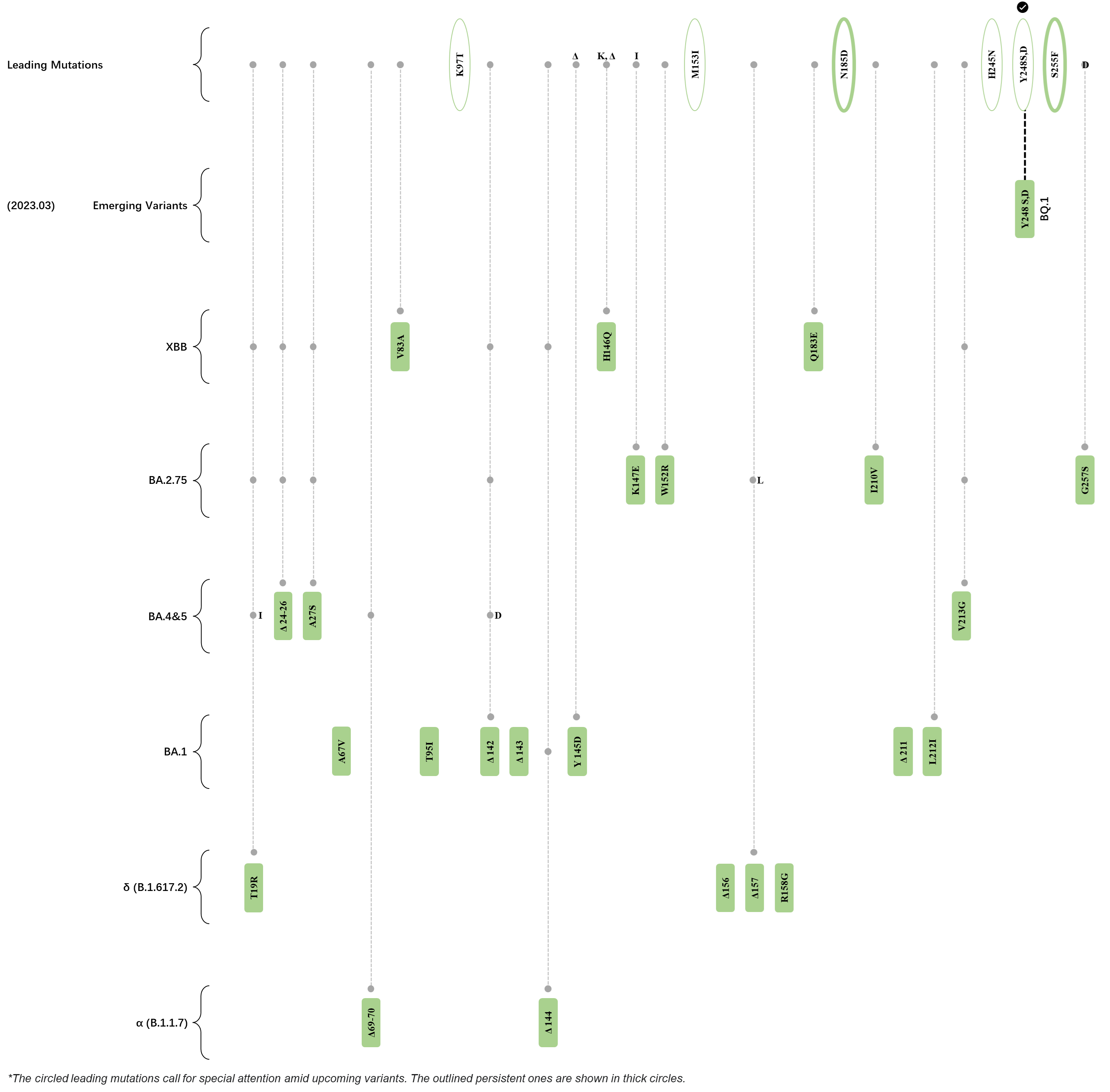
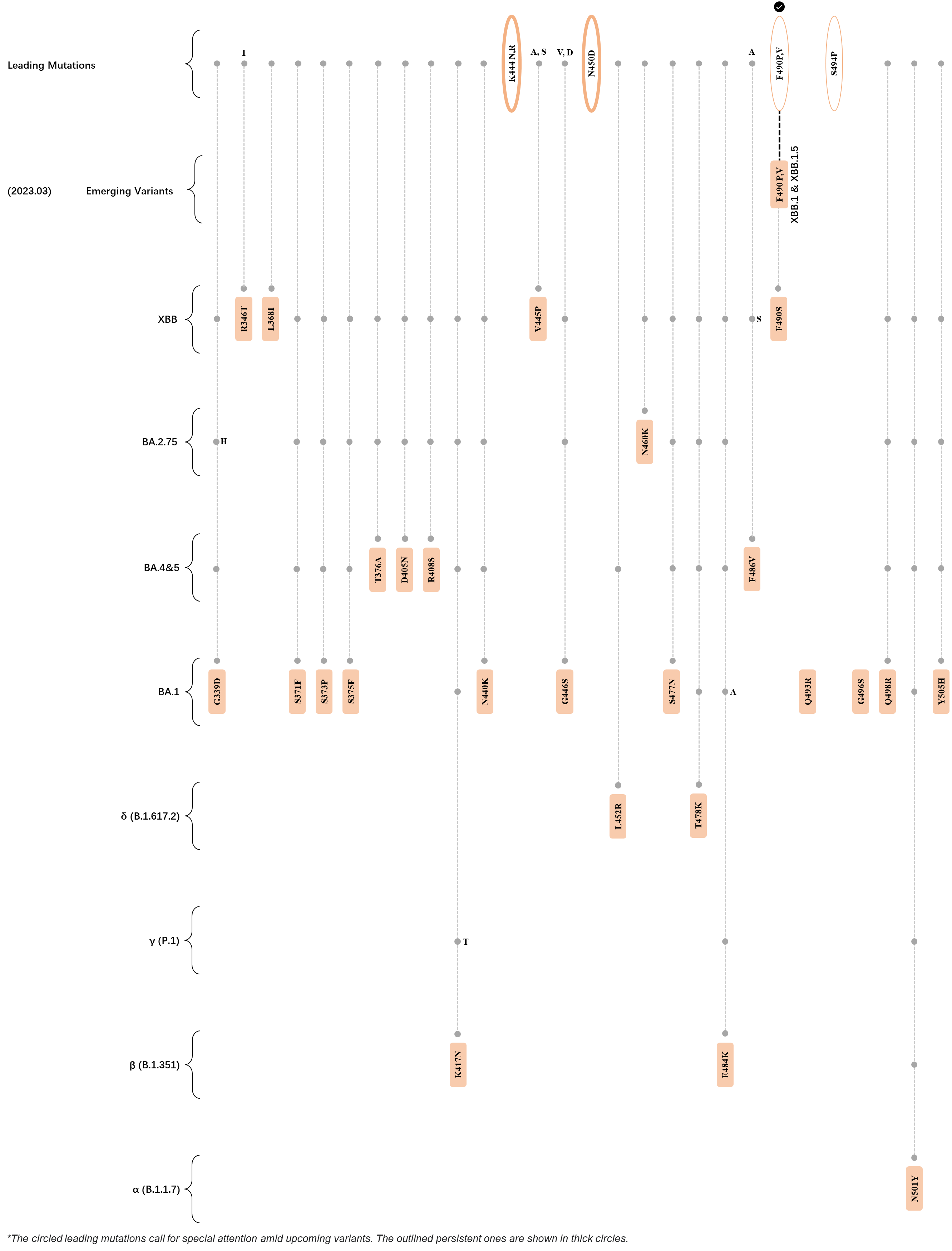
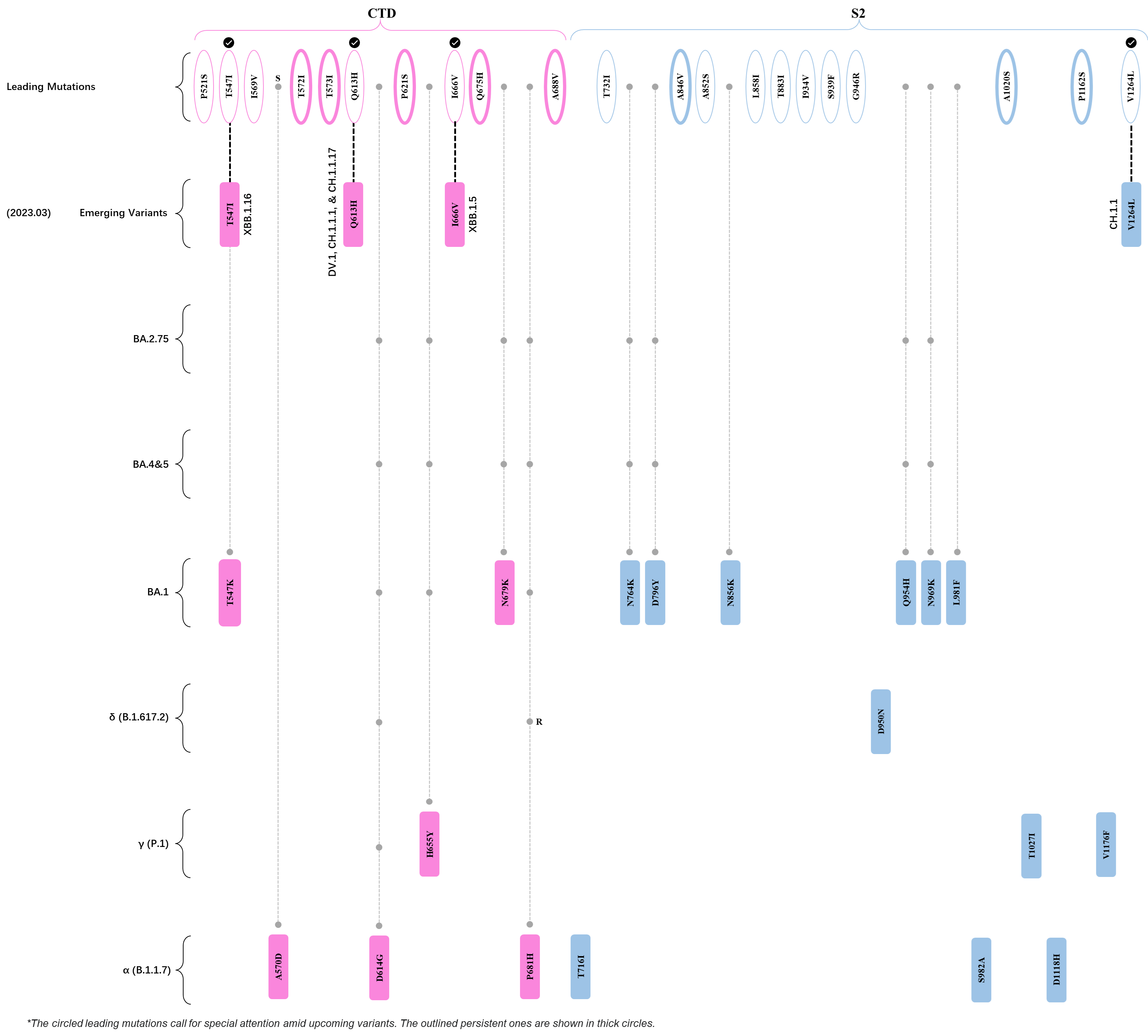
Click the button to show figure.

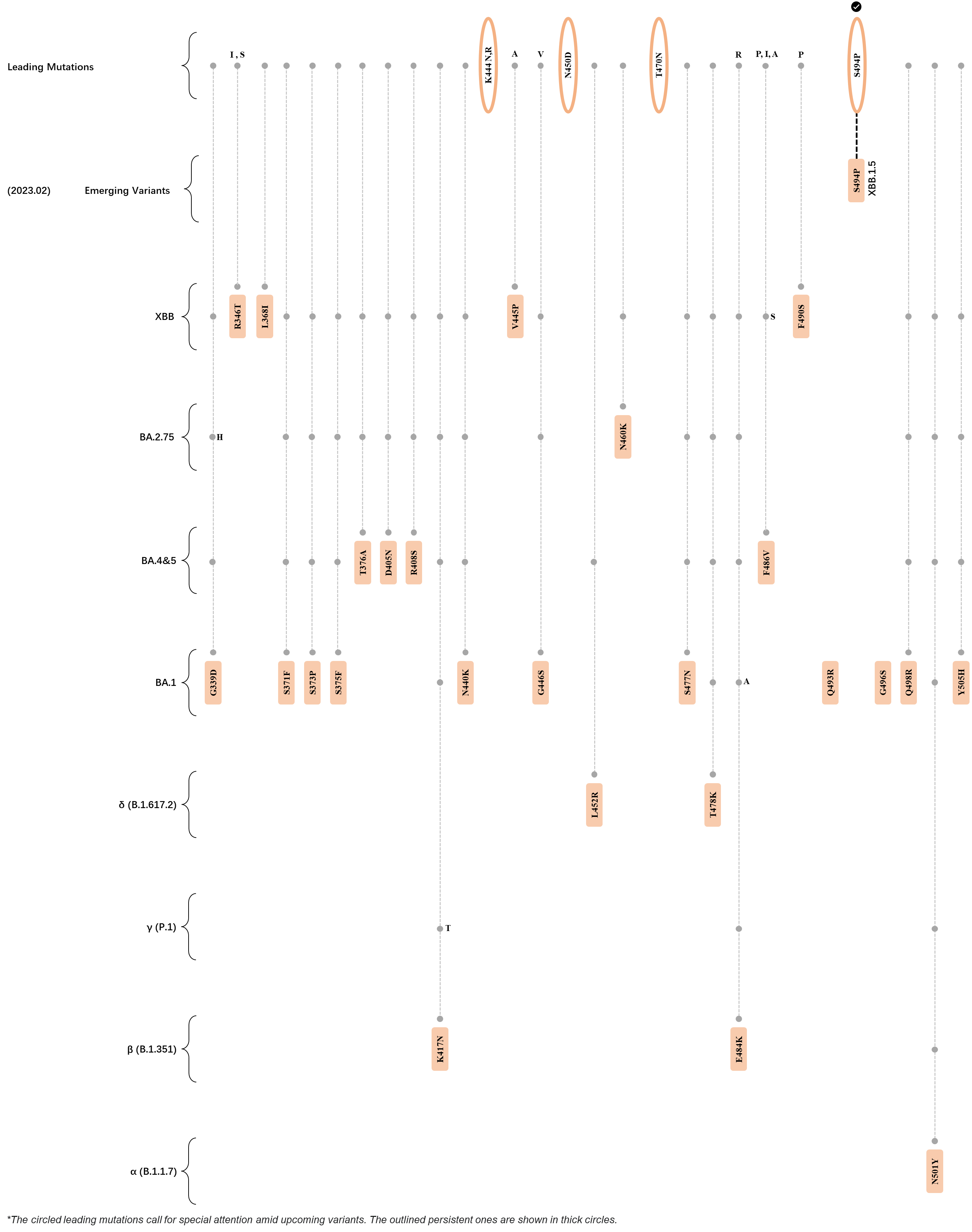

Click the button to show figure.
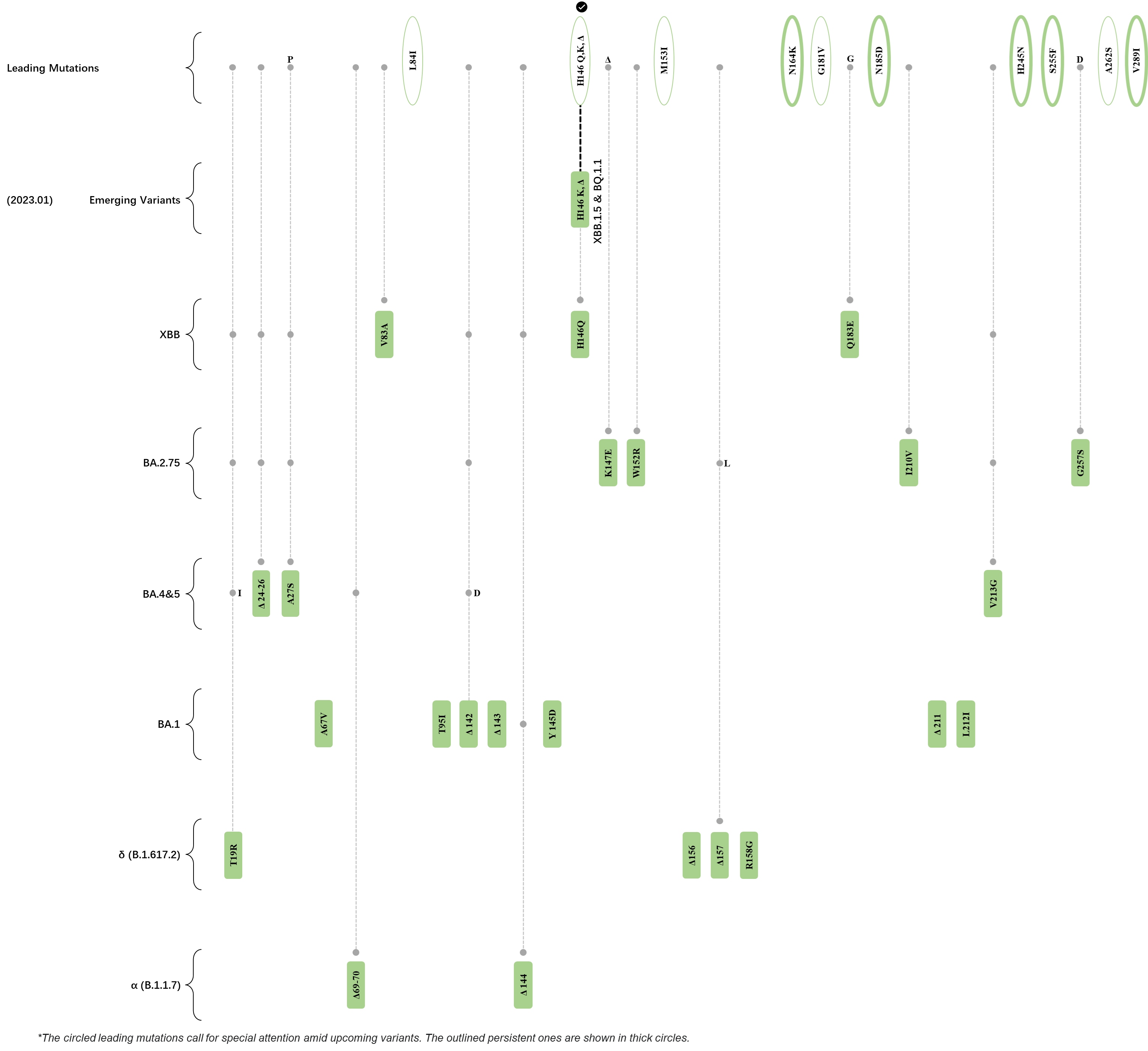
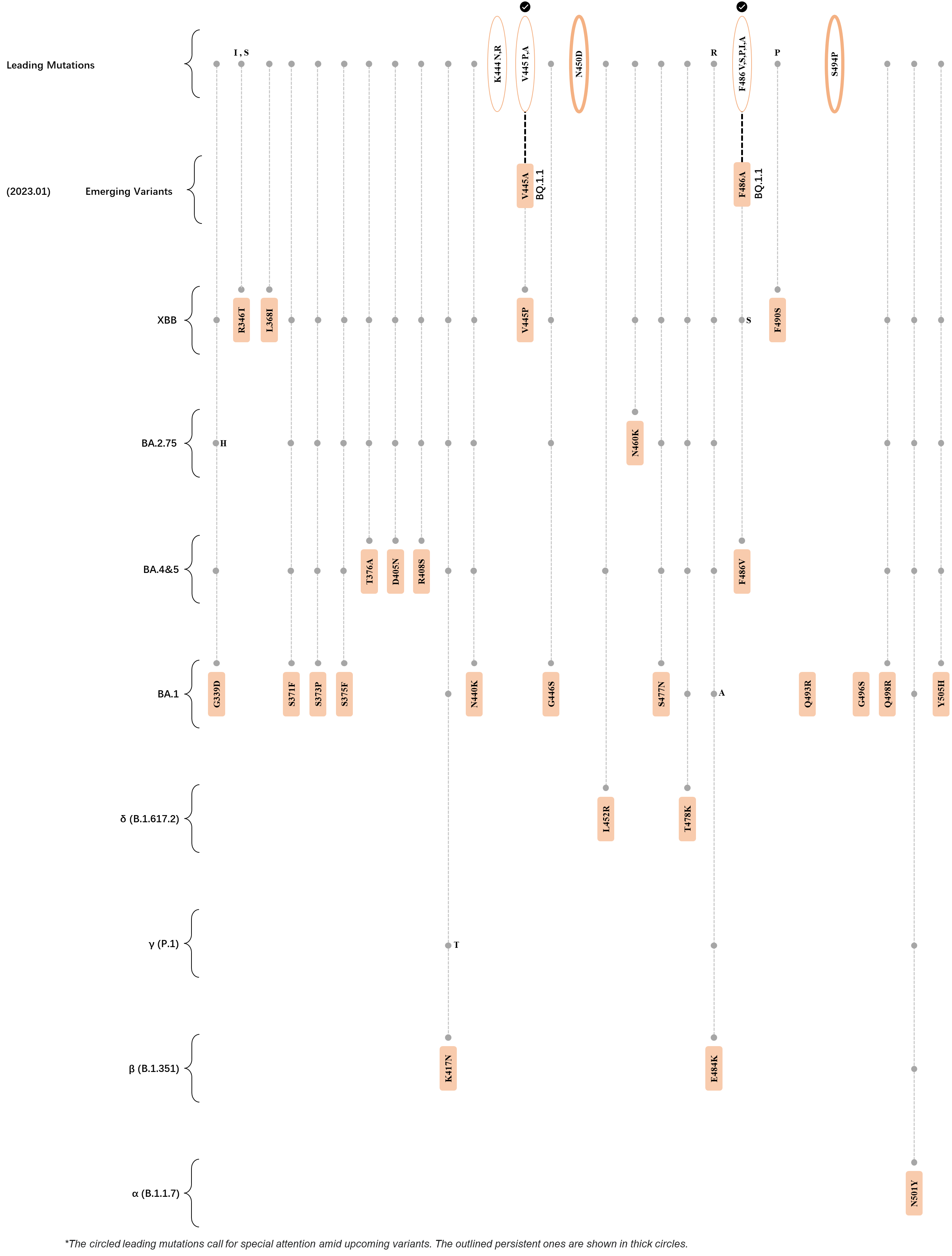
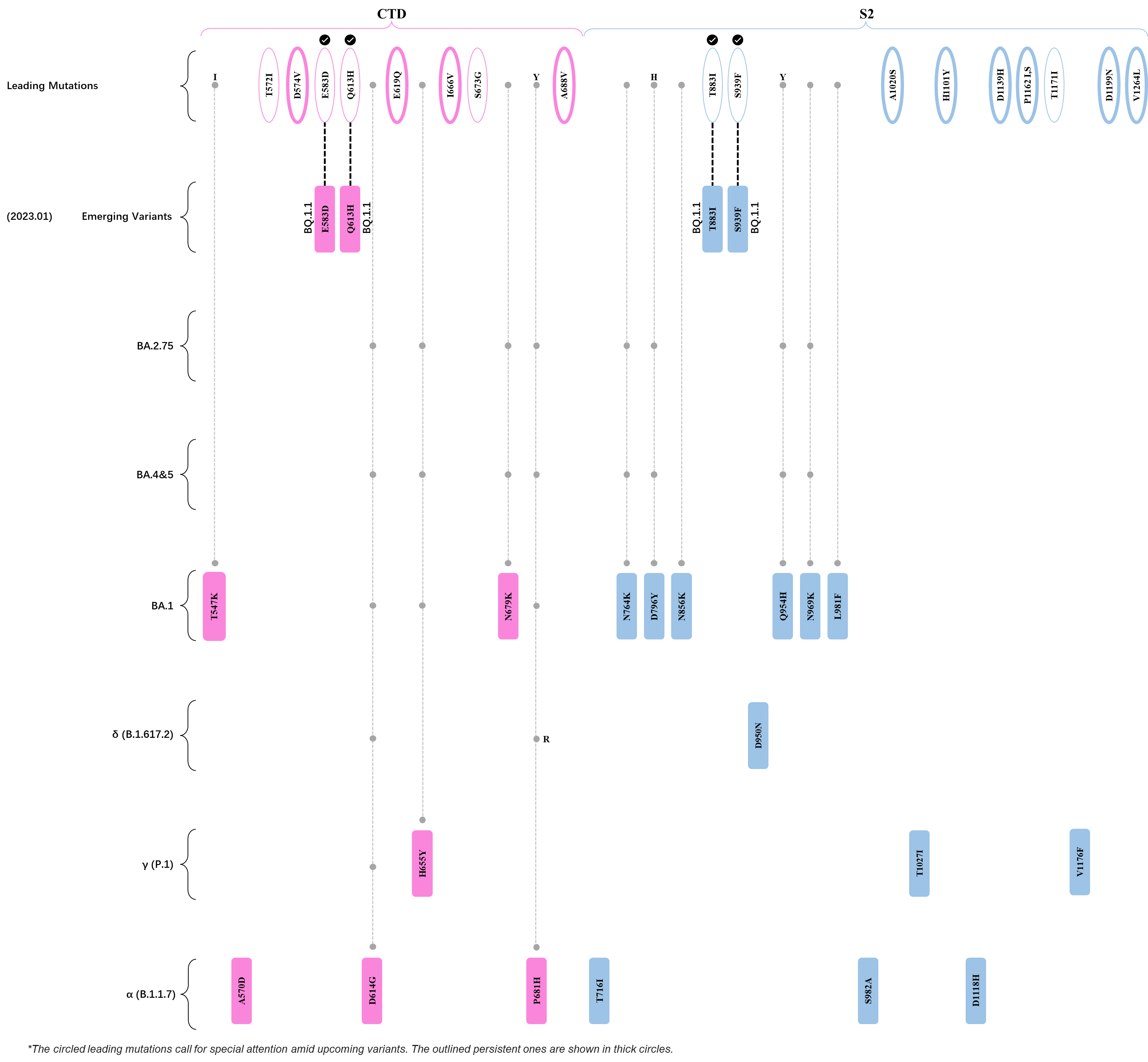
Click the button to show figure.


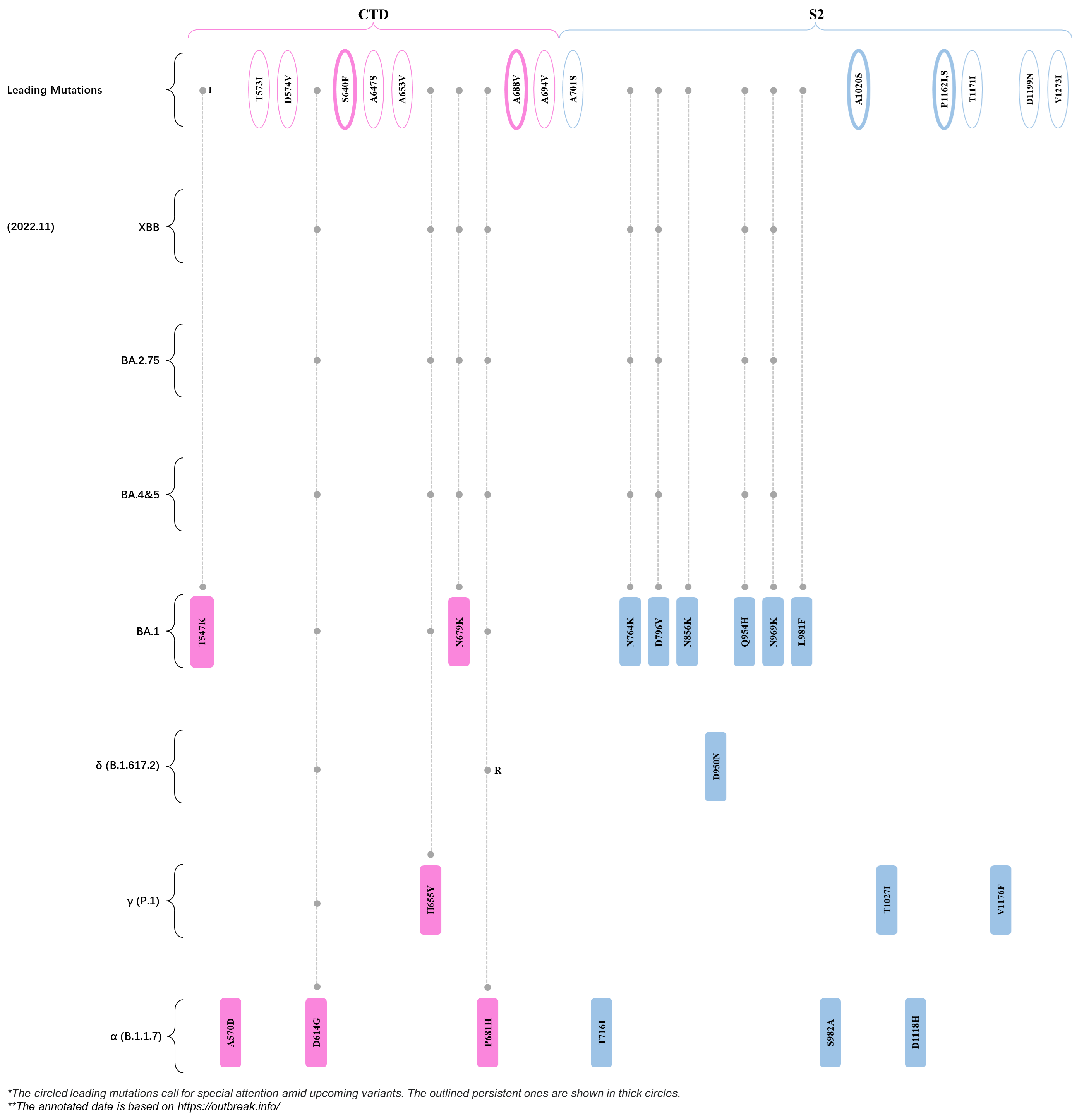
Click the button to show figure.
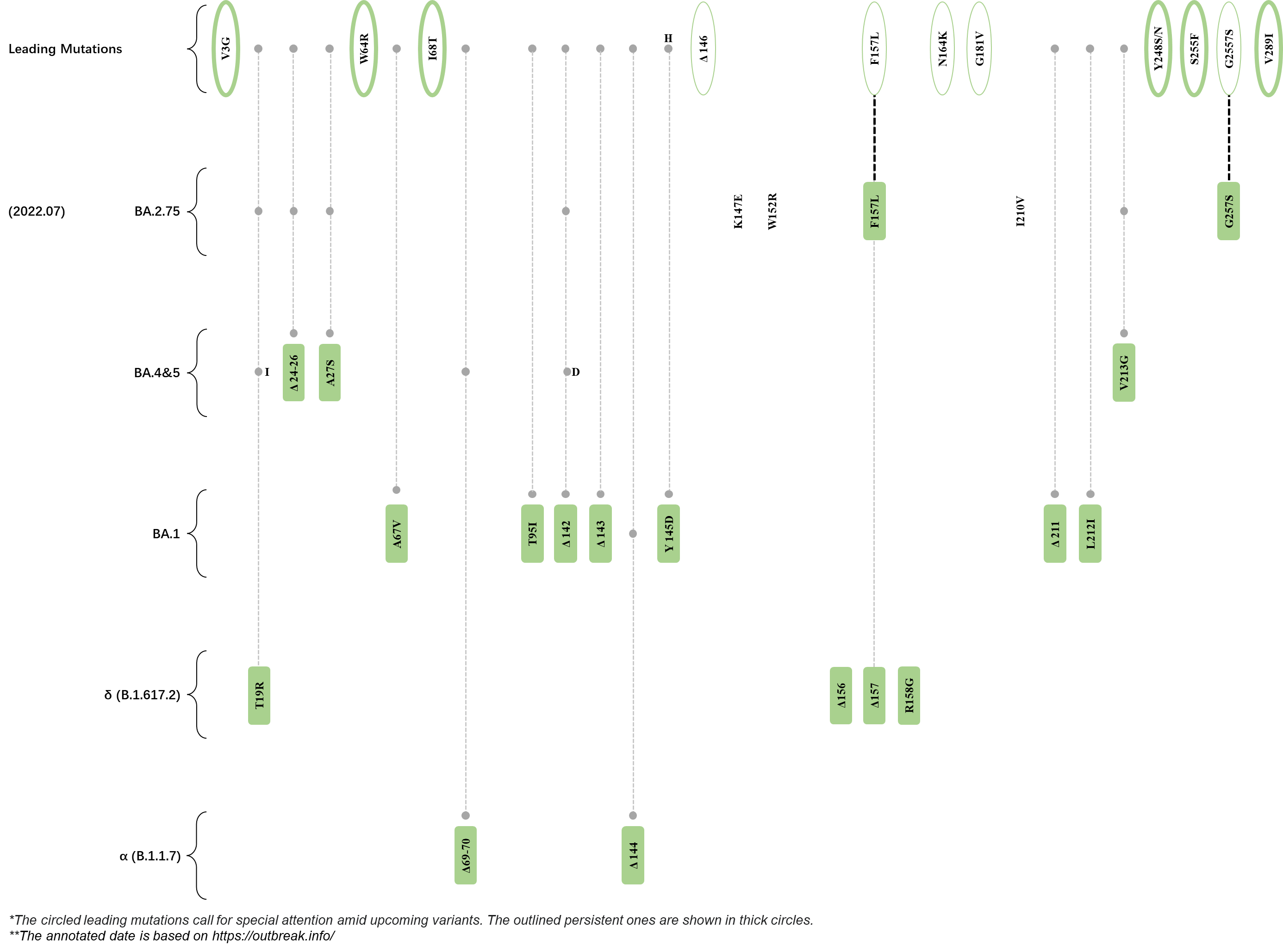
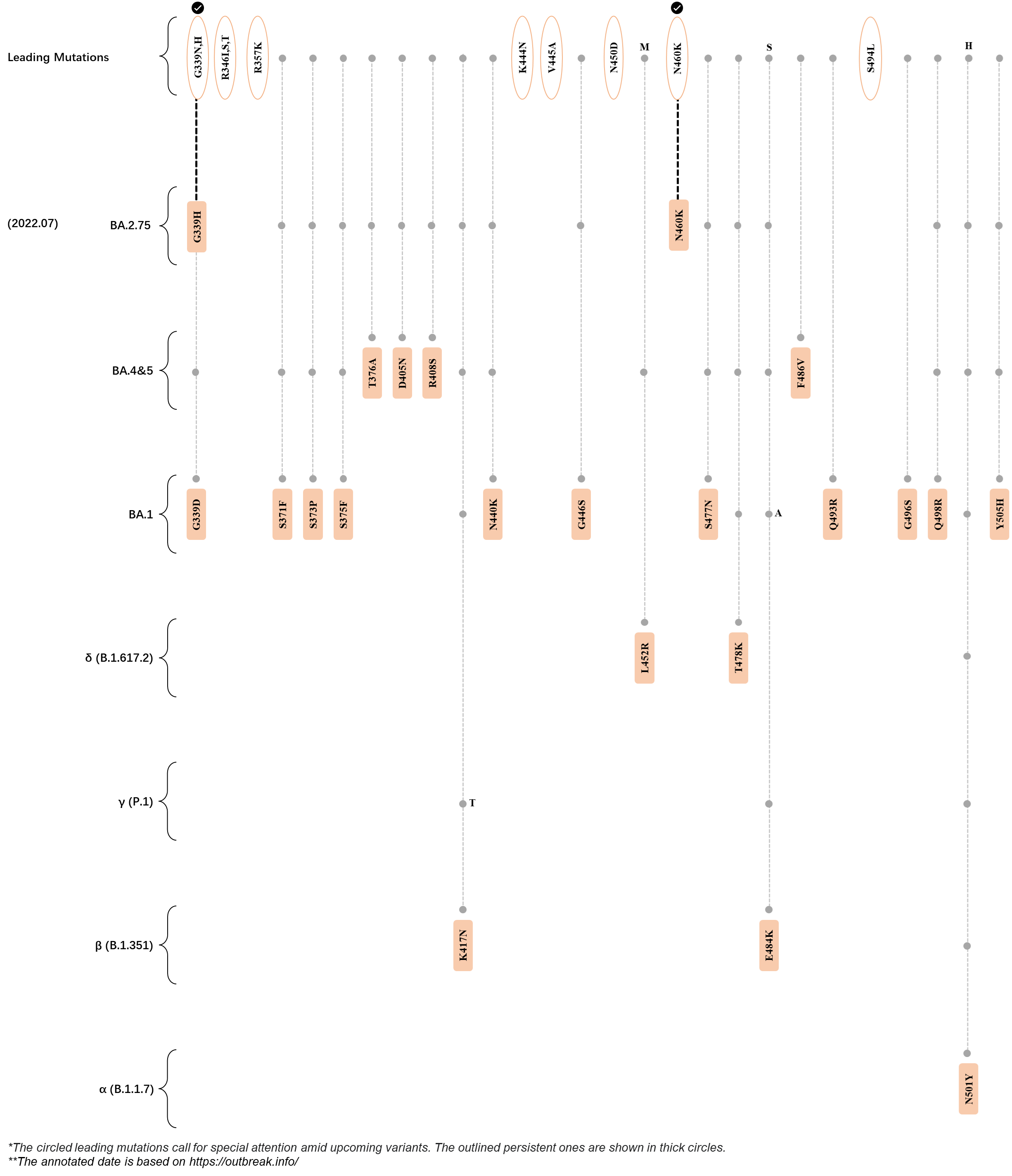
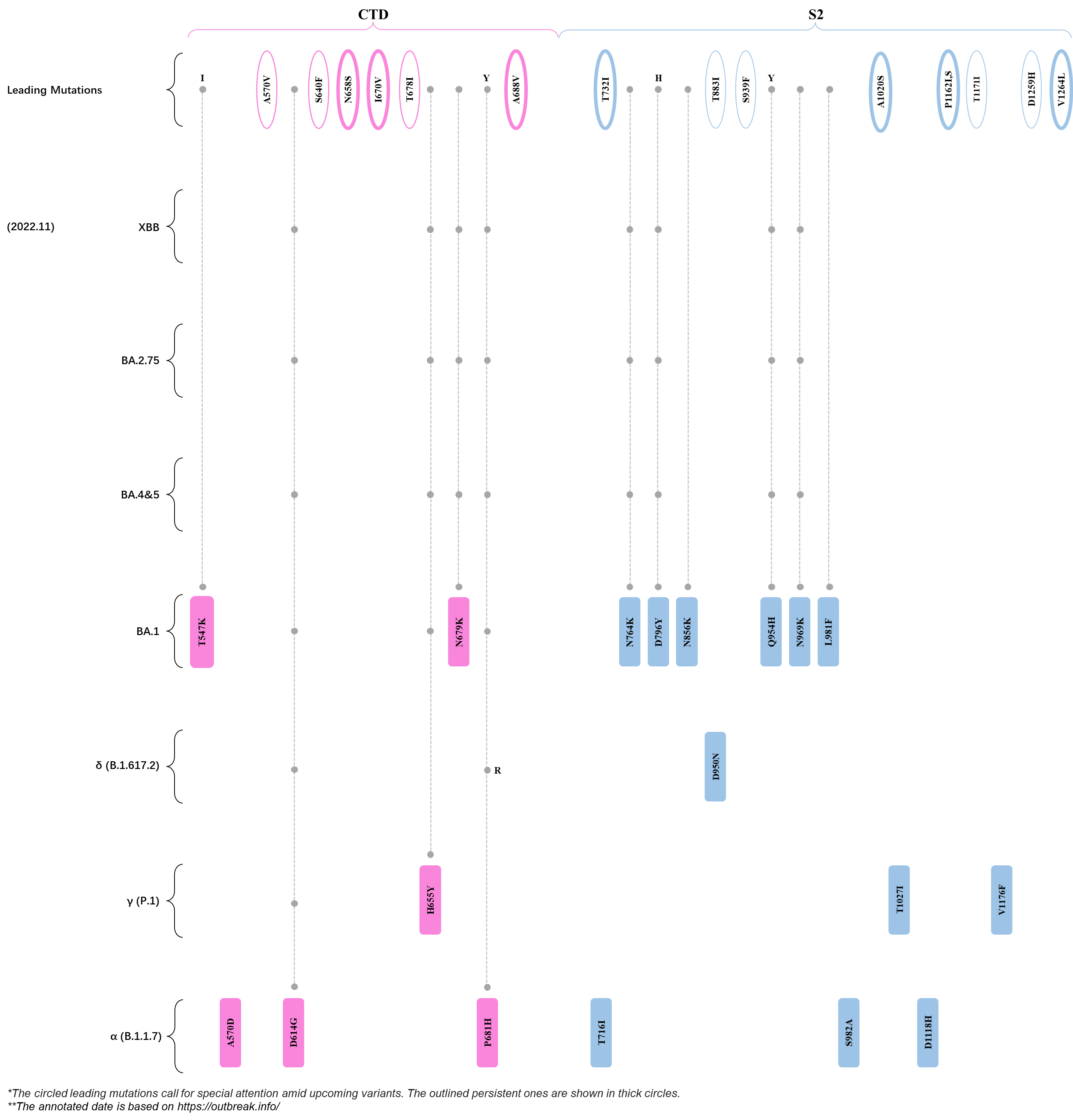
Click the button to show figure.
Click the button to show figure.